1. Introduction
Hepatocellular carcinoma (HCC) is one of the most lethal human cancers and the third leading cause of cancer mortality worldwide, resulting in some 600,000 deaths annually [
1]. Despite significant advances in surgery and radiotherapy, not all patients respond fully to therapeutic intervention. Furthermore, traditional chemotherapeutic drugs have several deficiencies including poor specificity, side effects and low tolerability. The enhancement of the host’s immune response has been recognized as a possible means of inhibiting tumor growth without harm to the host [
2]. Therefore, the study of novel natural biochemical materials that have anti-tumor activity and immunostimulatory properties has piqued the interests of many researchers.
Sea cucumbers are marine animals that are important as a human food source, particularly in some parts of Asia. They are typically soft-bodied echinoderms comprising a diverse group of flexible, elongated, worm-like organisms with leathery skin and a gelatinous body that resembles a cucumber [
3]. A multitude of harvestable sea cucumber species have been developed to meet the growing global demand for their food and pharmaceutical properties.
Stichopus japonicus acid mucopolysaccharide (SJAMP) is an important biologically active compound that is extracted from the body wall of the sea cucumber
Stichopus japonicus. SJAMP is made up of galactosamine, hexuronic acid, fucose and sulfuric acid with a proportion of 1:1:1:4 [
4,
5,
6]. SJAMP has multiple pharmacologic properties, including anti-tumor, immunologic regulation, anticoagulant and antiviral properties [
3]. Previous studies have shown that SJAMP has protective effects against several cancers and inhibits the proliferation of malignant cells through the induction of apoptosis [
7,
8]. To date, studies of the anti-tumor effect of SJAMP
in vitro have mainly focused on several different types of tumor cells, including HeLa, lung cancer, stomach cancer and liver cancer cells [
4,
7,
8]. However, the mechanisms of the anti-tumor activity of SJAMP remain largely unexplored.
There is a close relationship between immune functional status and the occurrence and progression of tumors [
9,
10,
11,
12]. Tumor cells that develop mechanisms against normal immune response, they may become pathogenic tumors, while down-regulation of the immune response in tumor-bearing patients may result in further tumor development, up-regulaton has been shown to be a useful therapeutic approach [
13]. Immunotherapy is a relatively novel approach to tumor therapy. However, numerous immune tolerogenic factors induced by viral infection or a chronic inflammatory response during hepatocyte carcinogenesis may accumulate, facilitating an aggressive and effective counterattack against the anti-HCC immunity of the host [
14]. In developing tumors anti-tumorigenic and pro-tumorigenic immune and inflammatory mechanisms coexist [
15]. Although the proinflammatory signaling cascades that mediate the pro-tumorigenic effects of inflammation are often activated during HCC development [
15,
16], immunomodulatory agents including those to release immune suppression and boost anti-HCC immunity are promising [
14].
In our current study, we therefore investigated the anti-tumor and immunomodulatory activities of SJAMP in a diethylnitrosamine (DEN)-induced HCC rat model. The results may help provide an experimental and theoretical basis for the study of the anti-tumor effects of SJAMP.
3. Experimental
3.1. Materials
A total of 50 Wistar mice (SPF grade, male, weighing 130–150 g) were purchased from the Shandong Lukang Laboratory Animal Center (Jining, China, SLXK-Lu20080002). SJAMP (purity > 99.5%) was provided by the College of Food Science and Engineering, Ocean University of China. The YAC-1 cell line was purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Optilyse C lysing solution was purchased from Immunotech (Marseille, France). FITC anti-rat CD3 antibody, PE anti-rat CD4 antibody and PE anti-rat CD8a antibody were purchased from Biolegend (San Diego, CA, USA). TNF-α and IL-2 enzyme-linked immunoabsorbent assay (ELISA) kits were obtained from Boster Biological Technology (Wuhan, China). Mouse anti-PNCA was purchased from ZSGB-BIO (Beijing, China). Mouse anti-p21 was purchased from Thermo (Waltham, MA, USA).
3.2. Experimental Schedules
Fifty rats were randomly divided into a normal control group, tumor control group, low-SJAMP-dose group, medium-SJAMP-dose group, and high-SJAMP-dose group (10 rats/group). All animal experiments were performed according to the rules and regulations of the Animal Ethics Committee of Qingdao University Medical College, China. Rats in the normal control group were fed a normal diet. Rats in the tumor control group and SJAMP intervention groups received an intragastric administration of a 2% DEN saline solution (10 mg/kg of body weight) once daily for 5 days/week for a period of 15 weeks. Concurrently, the low-, medium- and high-SJAMP-dose groups were given 17.5, 35, and 70 mg/kg/day of SJAMP, respectively. The tumor control group received intragastric administration of an equivalent amount of normal saline. Rats were weighed once a week. At the 16th week, all rats were anesthetized with chloral hydrate, and the abdominal aortic blood, liver tissues, spleen and thymus were separated and weighed.
Macroscopically visible nodules greater than 3 mm or 5 mm in diameter on the liver surface were recorded. The longest and shortest diameters of the largest tumor nodules were measured using a vernier caliper. Volume of the largest nodules was estimated using the following equation [
34]:
where a is the longest axis, and b is the shortest axis).
3.3. Measurement of Liver Function Parameters, Serum AFP, TNF-α and IL-2 Levels
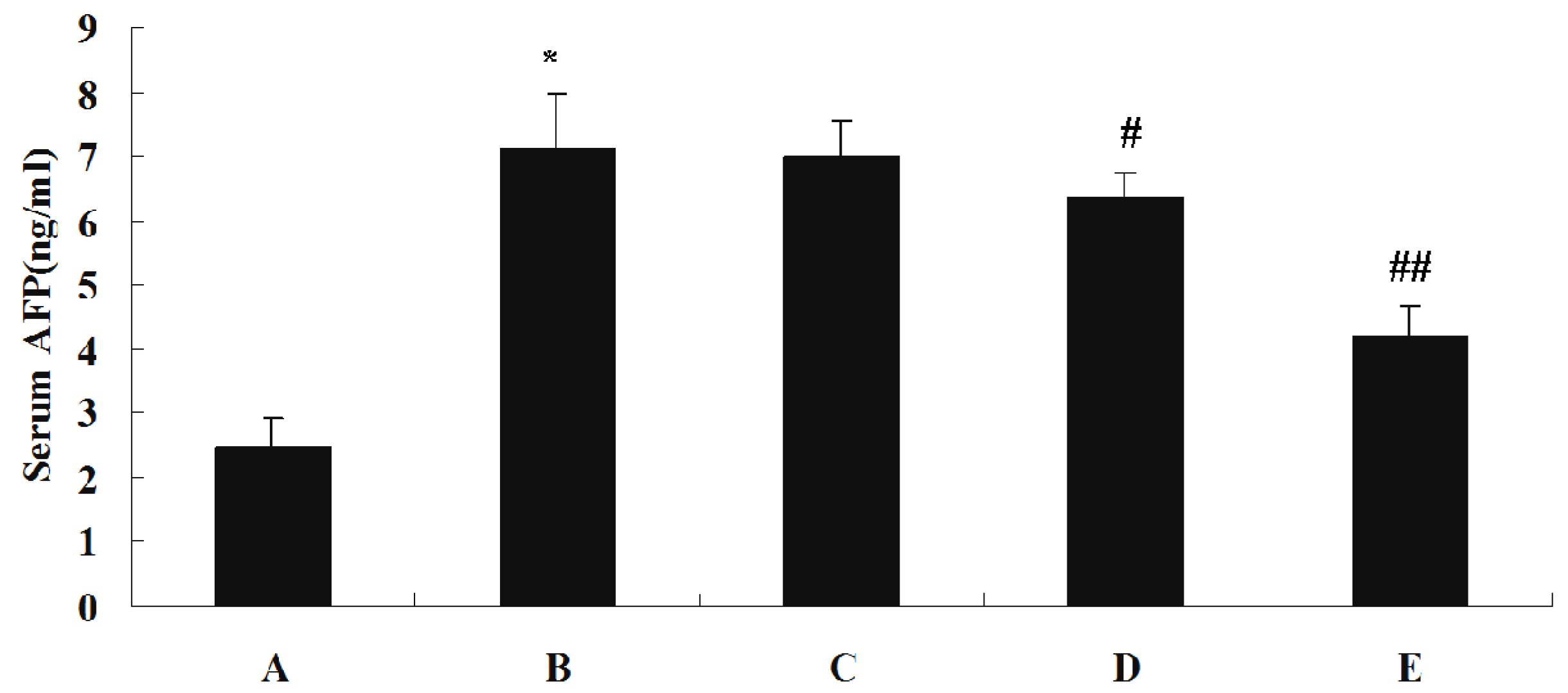
Levels of ALT, AST and GGT in each serum sample were measured using a Hitachi 7170A Modular Analytic system. Serum concentrations of AFP, TNF-α and IL-2 were assayed by the ELISA method using commercially available enzyme immunoassay kits, according to the manufacturer specifications.
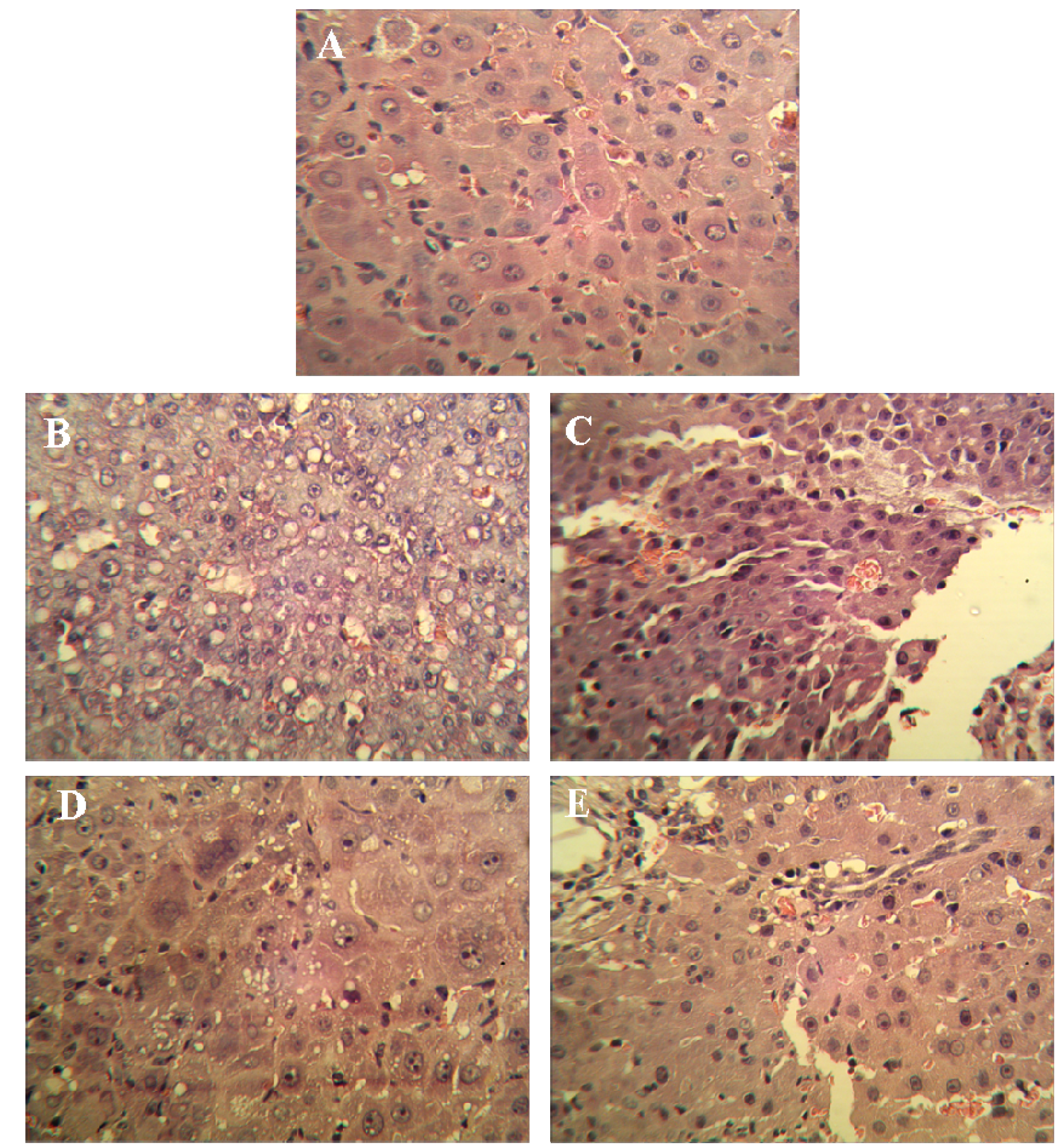
3.4. Pathological Histology and Immunohistochemistry
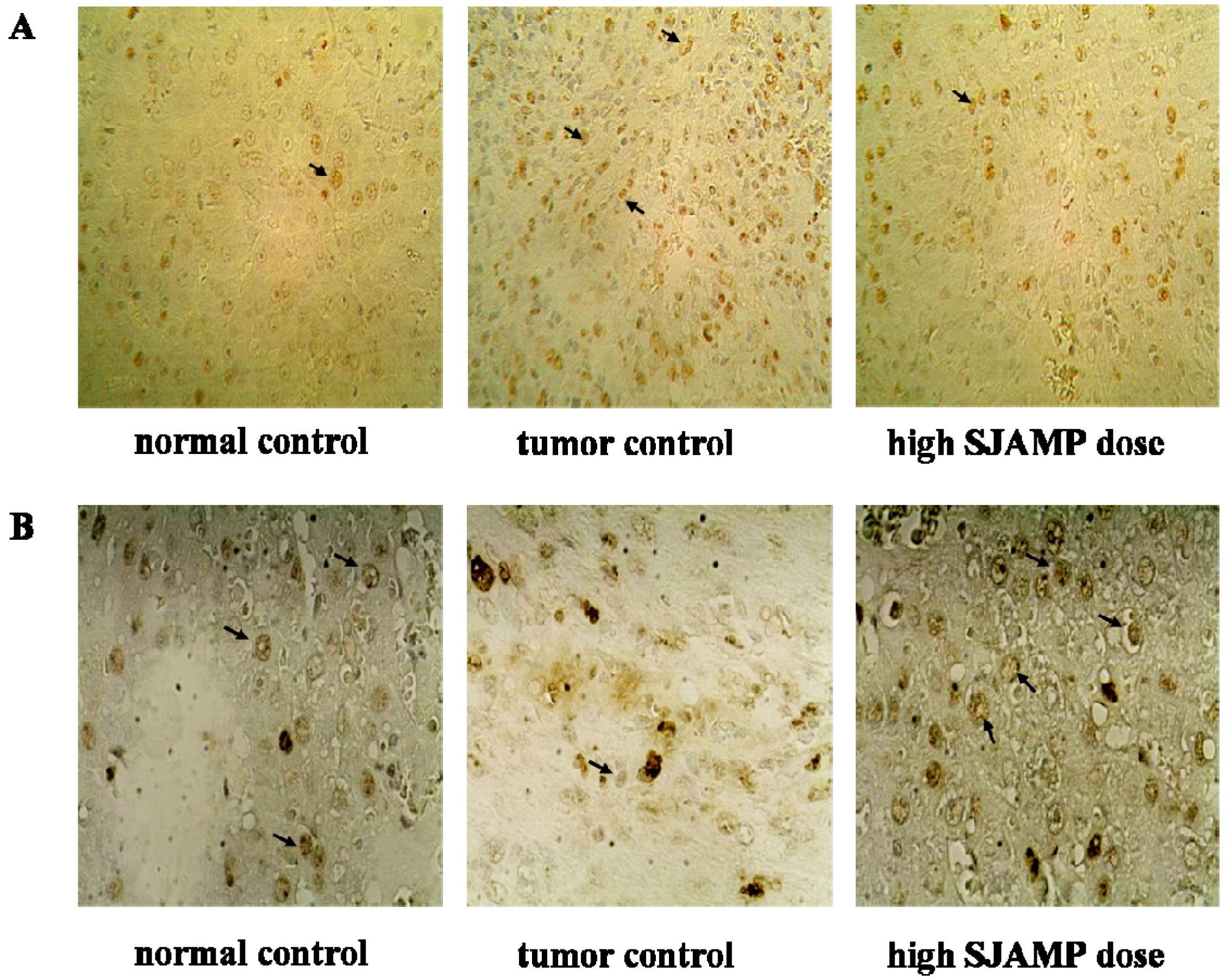
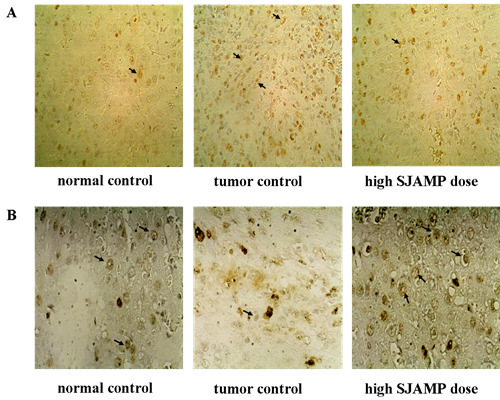
Liver tissues were collected, embedded in paraffin, cut into slices and stained with hematoxylin and eosin (H & E) for histopathological examination under a microscope. Paraffin-embedded, 5 μm-thin sections from liver specimens were deparaffinized, blocked with 0.3% hydrogen peroxide for 10 min and subjected to antigen retrieval in a steamer for 20 min. The slides were then washed twice with PBS and incubated with primary antibodies of PCNA and p21. Incubation with an appropriate secondary antibody was followed by direct DAB staining and light counterstaining with hematoxylin. Staining procedures strictly followed the supplier’s recommendations. The proliferation index was determined as the percentage of positively stained nuclei by counting 1000 cells in field at magnification × 200. p21 was quantified by counting positively stained cells and total number of cells at 10 randomly selected fields from each section at 400 × magnifications, and data are presented as a percentage.
3.5. Preparation of Spleen Cell Suspensions
The spleens of the experimental animals were washed twice with D-Hank’s solution to remove blood and other non-specific tissues. Approximately 0.5 cm3 of spleen was split and ground, and the spleen cells were resuspended. Cells were washed twice with D-Hank’s solution, and the supernatants were discarded. Five mL of an NH4Cl lysis solution was then added to lyse the erythrocytes. After centrifuging the suspensions at 1,000 rpm for 15 min at 4 °C, the remaining cells were washed twice with PBS and then resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum. The cells were then seeded into culture bottles and cultured for 2 h in a CO2 incubator at 5% CO2. The vast majority of the adherent cells were macrophages, and the non-adherent cells were mainly mixed spleen lymphocytes.
3.6. Determination of the Phagocytic Activity of the Macrophages
Phagocytic ability of macrophages was measured via neutral red dye uptake [
35]. Adherent macrophages were released into single-cell suspensions using a 0.25% trypsin digestion and adjusting the cell density to 1 × 10
6 cells/mL. An aliquot of 200 μL of the cell suspension was added to each of the wells of a 96-well plate (each sample had three parallel wells) and cultured for 48 h. One hundred μL of the culture medium was then aspirated from each well, 100 μL of a 0.1% neutral red solution was added to each well, and the plate was incubated for 30 min at 37 °C in a 5% CO
2 humidified incubator. Finally, the plate was washed three times with PBS, and 100 μL of lysis buffer (0.1 mol/L acetic acid:ethanol = 1:1) was added to each well. The treated plates were stored at 4 °C in the refrigerator overnight, and the OD value at 492 nm was determined using an enzyme-labeled instrument.
3.7. Assessment of NK Cell Cytotoxicity
A lactate-dehydrogenase (LDH)-release assay was used to measure the NK cell-mediated tumor cytotoxicity. Spleen cells from each rat, prepared as previously described, were used as the effector cells. The NK-sensitive cell line YAC-1 was used as the target cell. Two types of cell suspension were prepared that contained 95% viable cells based on staining determinations with trypan blue. A 200 μL cell suspension that including 100 μL of the effector cell suspension (1 × 10
6/mL) and 100 μL of the target cell suspension (1 × 10
5/mL) was added into the wells of 96-well plates (each sample had three parallel wells). After a 4-h incubation at 37 °C in a 5% CO
2 humidified incubator, the plates were centrifuged at 1,500 rpm for 15 min. The supernatants from each well (100 μL) were transferred into the corresponding wells of another 96-well plate. One hundred μL of lactic acid hydrogenase substrate mixture was then added to each well. After 6 min, the reactions were stopped with 1 mol/L HCl. The OD value of each well was measured at 492 nm. The cytotoxic activity of the NK cells was estimated using the following equation:
where
E represents the experimental release of LDH activity from the target cells incubated in the presence of the effector cells,
S is the spontaneous release of the LDH activity from the target cells alone, and
M represents the maximum release of the LDH activity determined by lysing the target cells with 1% NP-40.
3.8. T-lymphocyte Subsets in Rat Peripheral Blood Flow Cytometric Analyses
Aliquots of 100 μL of peripheral blood from each rat were added to Eppendorf tubes containing pre-added monoclonal antibodies against CD3, CD4 and CD8a (anti-CD3-FITC, anti-CD4-PE and anti-CD8-PE) and incubated for 15 min at room temperature in the dark. The red blood cells were lysed using Optilyse C lysing solution following the manufacturer’s instructions. After centrifuging at 1,000 rpm for 10 min at room temperature in the dark, the samples were washed twice with PBS. Then, the cell sediments were suspended in 0.5 mL PBS. The prepared suspensions were analyzed on a Cytomics FC 500 flow cytometer (Beckman Coulter). A total of 20,000 cells were analyzed for each sample.
3.9. Statistics
All data are expressed as the means ± SD. The data were statistically analyzed using one-way analysis of variance (ANOVA). Post-hoc comparisons were carried out by the least-significant difference (LSD) test. Probability values lower than 0.05 were considered significant.