Self-Assembly of a 1D Hydrogen-Bonded Polymer from a Hexamethyltetraaza Macrocyclic Nickel(II) Complex and Isophthalic Acid
Abstract
:1. Introduction
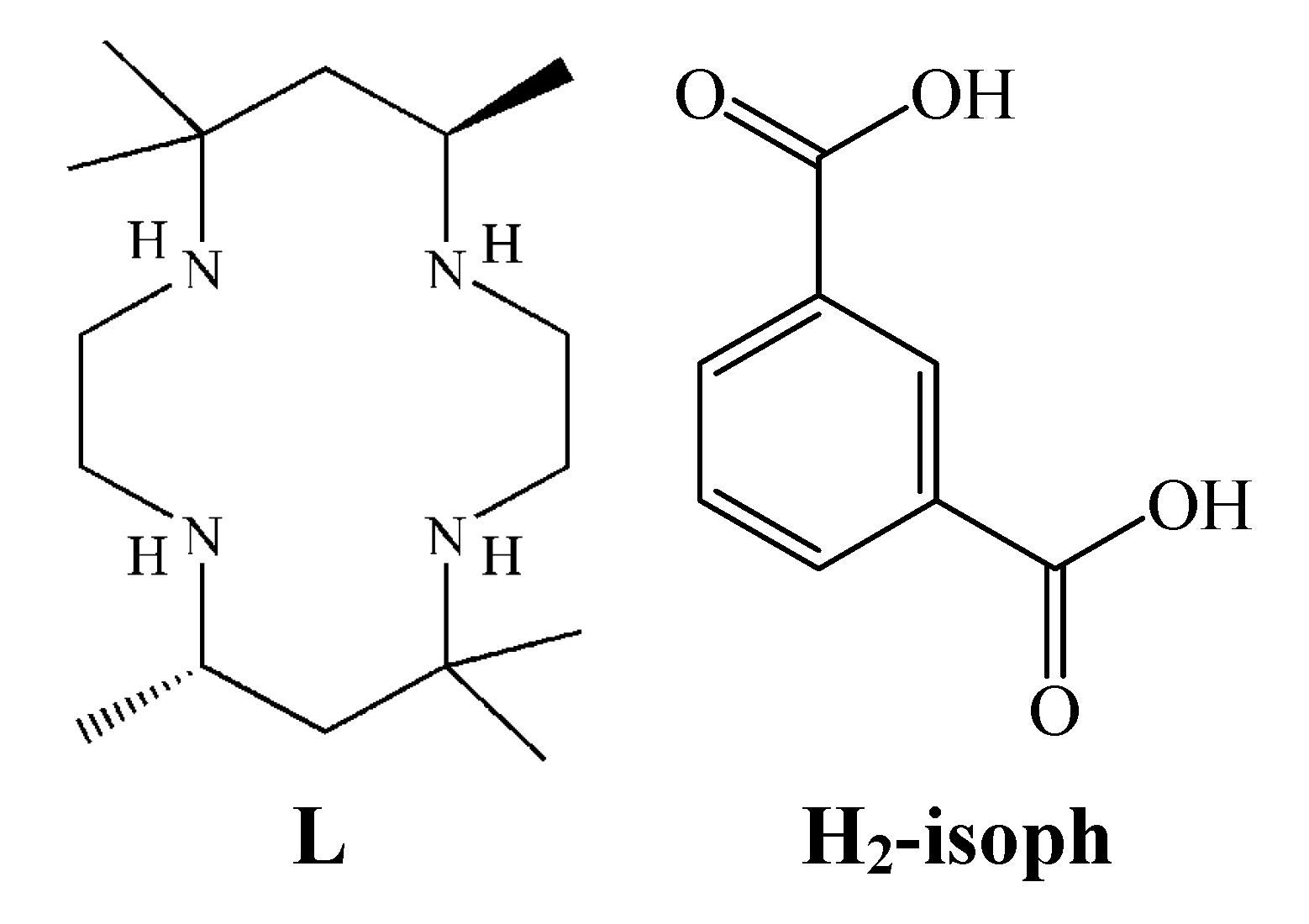
2. Results and Discussion
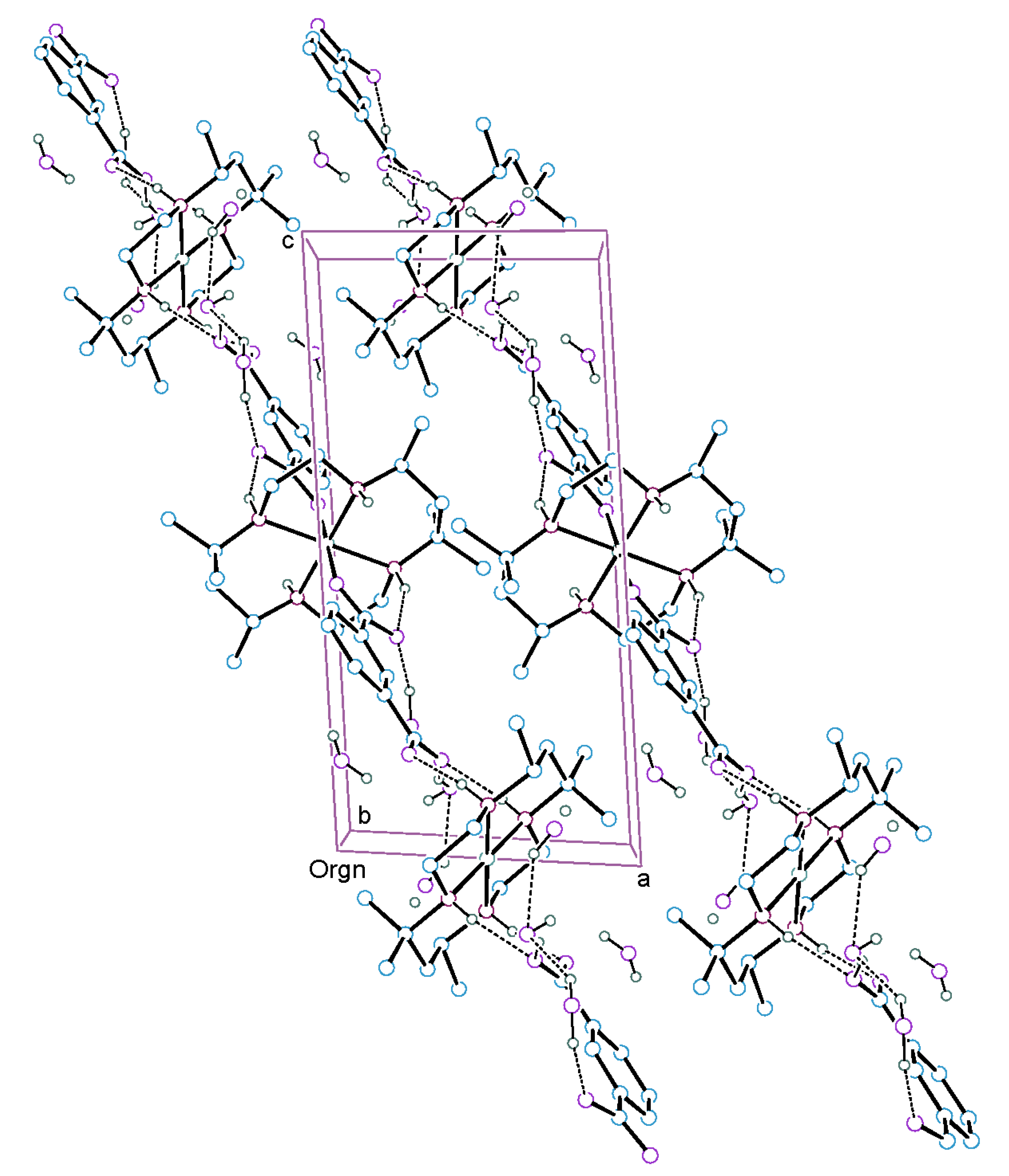
2.1. Description of the Structure

| Bond lengths and angles | |||
|---|---|---|---|
| Bond lengths | |||
| Ni(1)-N(1) | 2.130(7) | Ni(1)-N(2) | 2.009(7) |
| Ni(1)-O(1) | 2.056(6) | Ni(2)-N(3) | 1.913(7) |
| Ni(2)-N(4) | 1.878(8) | C(9)-O(1) | 1.254(11) |
| C(9)-O(1) | 1.238(11) | C(16)-O(3) | 1.196(12) |
| C(16)-O(4) | 1.239(12) | Ni(1)…Ni(2) | 11.641(8) |
| Ni(1)…Ni(2)i | 9.607(4) | ||
| Bond angles | |||
| N(1)-Ni(1)-N(2) | 87.0(3) | N(1)-Ni(1)-N(2)iii | 93.0(3) |
| N(1)-Ni(1)-O(1) | 89.7(3) | N(1)iii-Ni(1)-O(1) | 90.3(3) |
| N(2)-Ni(1)-O(1) | 85.6(3) | N(2)iii-Ni(1)-O(1) | 94.4(3) |
| N(3)-Ni(2)-N(4) | 91.3(4) | N(3)-Ni(2)-N(4)iv | 88.7(4) |
| Ni(1)-O(1)-C(9) | 133.7(6) | O(1)-C(9)-O(2) | 127.7(8) |
| O(3)-C(16)-O(4) | 122.6(9) | ||
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A (°) |
|---|---|---|---|---|
| N(1)-H(1)…O(2)iii | 0.91 | 1.95 | 2.804(10) | 154.6 |
| N(3)-H(3)…O(4) | 0.91 | 1.98 | 2.869(10) | 164.0 |
| N(4)-H(4)…O(3) | 0.91 | 1.98 | 2.841(11) | 157.8 |
| Ow(1)-HO(1)…O(4) | 1.07(10) | 1.65(11) | 2.676(11) | 160(9) |
| Ow(2)-HO(3)…O(2) | 1.00(11) | 1.76(11) | 2.734(12) | 163(9) |
| Ow(2)-HO(4)…Ow(1) | 0.89(13) | 1.92(13) | 2.706(14) | 147(11) |
| Ow(4)-HO(8)…Ow(1)iv | 0.82(13) | 2.12(14) | 2.829(17) | 145(13) |
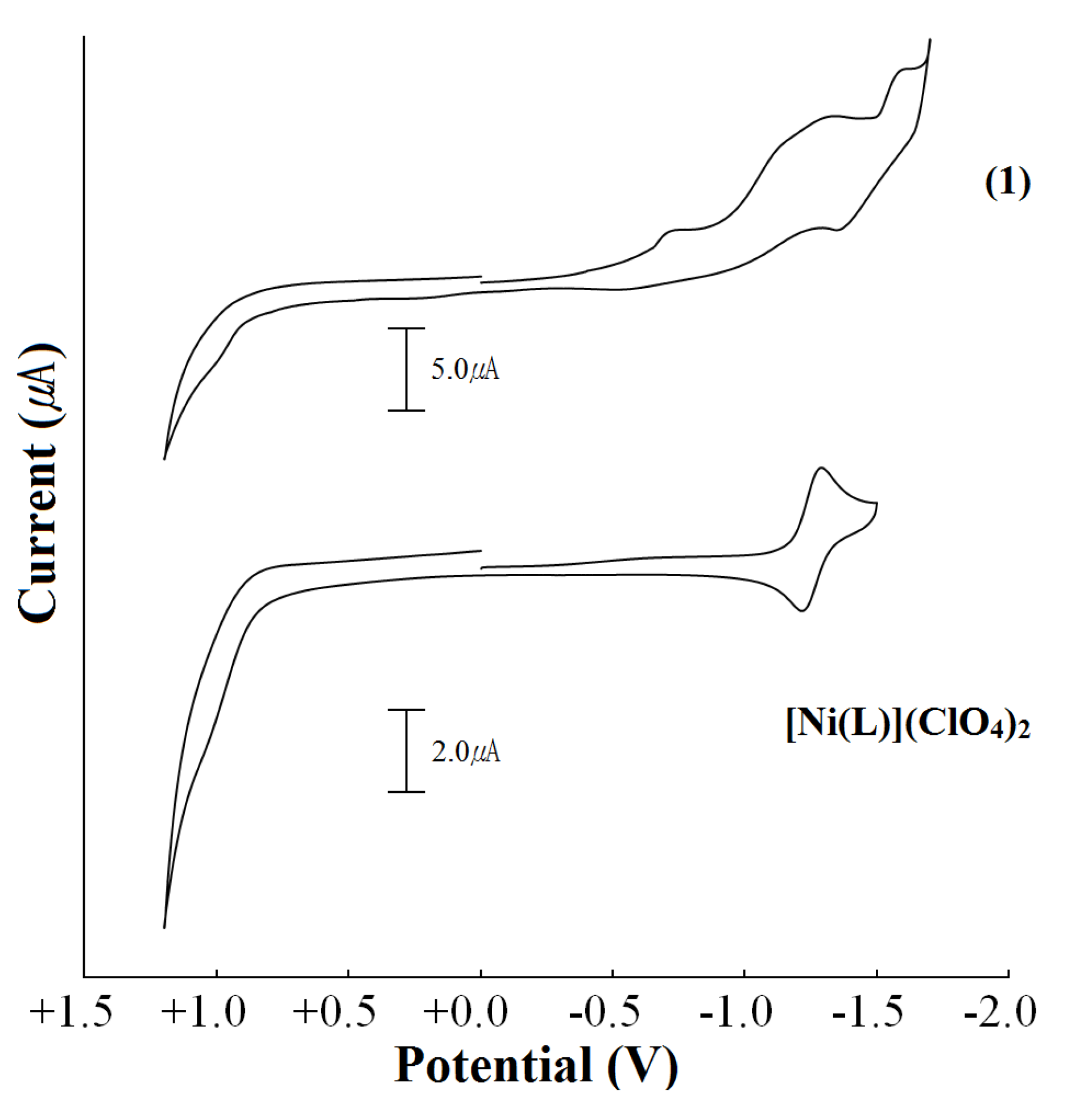
2.2. Chemical Properties
| Complex | State | λmax/nm (ε/M−1 cm−1) |
|---|---|---|
| [Ni(L)](ClO4)2 b | H2O | 463(80) |
| CH3CN | 469(63) | |
| Solid | 262, 468 | |
| [Ni(L)(isoph)2][Ni(L)]∙8H2O (1) | H2O | 260(5.2 × 103), 458(71) |
| Solid | 325(sh), 474, 696 |

| Complex | Potentials(V) Ag/AgCl |
|---|---|
| Ni(II)/Ni(I) | |
| [Ni(L)](ClO4)2 b,a | −1.29 |
| [Ni(L)(isoph)2][Ni(L)]∙8H2O (1) | −0.76(i) c |
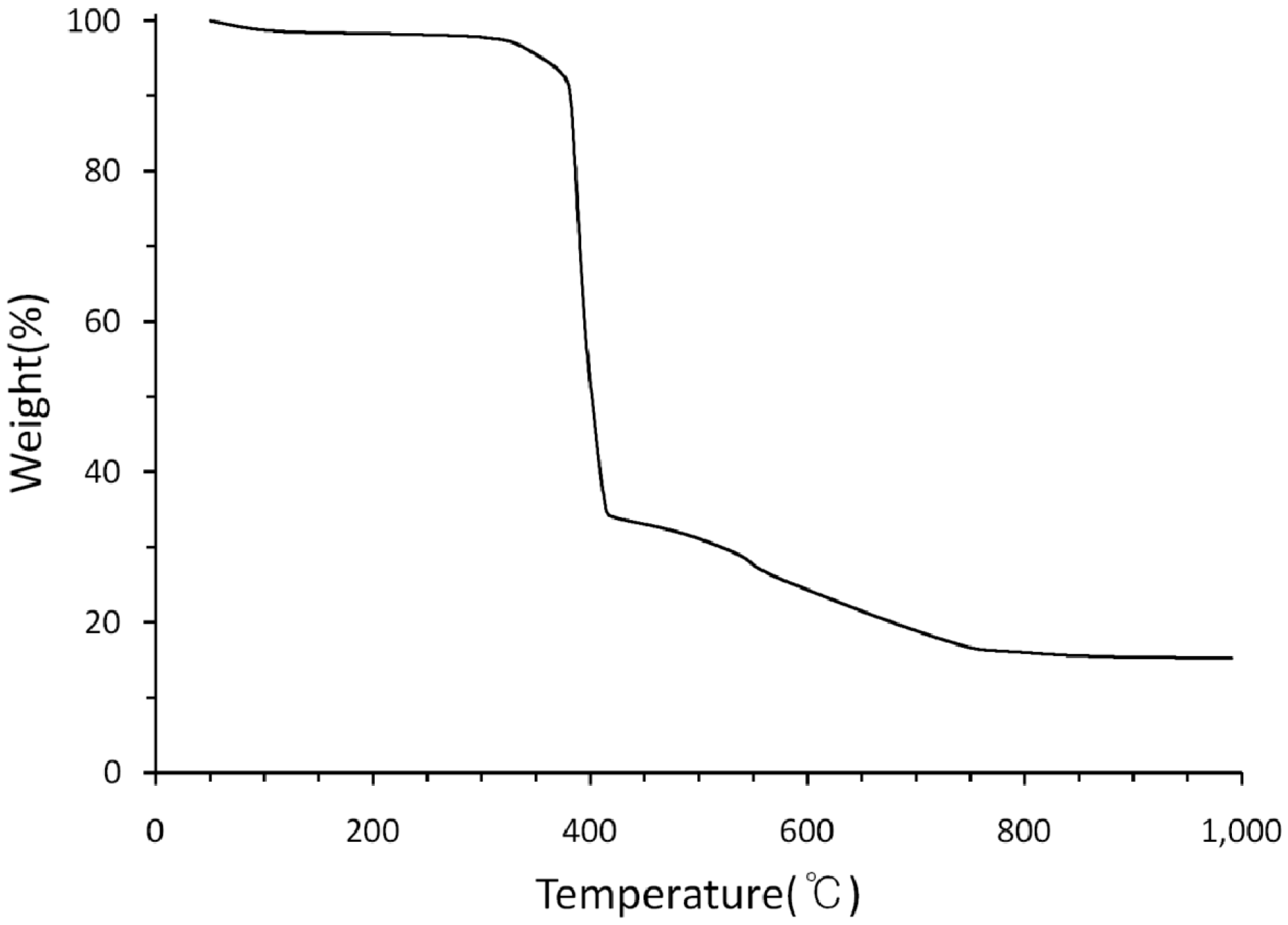
3. Experimental
3.1. Materials and Physical Measurements
3.2. Synthesis of [Ni(L)(isoph)2][Ni(L)]·8H2O (1)
3.3. X-ray Crystallography
| Data and refinement | |
|---|---|
| Empirical formula | C48H96N8Ni2O16 |
| Formula weight | 1157.94 |
| Temperature (K) | 293(2) |
| Crystal color/habit | Brown/prism |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | |
| a (Å) | 8.602(2) |
| b (Å) | 10.684(7) |
| c (Å) | 16.550(3) |
| α (°) | 91.04(4) |
| β (°) | 94.09(2) |
| γ (°) | 111.09(4) |
| V (Å3) | 1413.9(10) |
| Z | 1 |
| Dcalc (Mg m−3) | 1.360 |
| Absorption coefficient (mm−1) | 0.738 |
| F(000) | 624 |
| Crystal size (mm3) | 0.40 × 0.30 × 0.10 |
| θ range (°) | 1.23 to 26.37 |
| Limiting indices | −10 ≤ h ≤ 10, −13 ≤ k≤ 13, −1 ≤ l≤ 19 |
| Reflection collected/unique | 5729/5137 (Rint = 0.0817) |
| Absorption correction | ψ-scan |
| Max./min. transmission | 0.9145 and 0.7122 |
| Data/restraints/parameters | 5137/0/369 |
| Goodness of fit on F2 | 1.184 |
| Final R indices (I>2σ(I)) | R1a = 0.0811, wR2b = 0.2224 |
| R indices (all data) | R1 = 0.1742, wR2 = 0.3391 |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.20000P)2 +0.0000P] |
| rgest difference peak and hole (eÅ−3) | with P = (Fo2 + 2Fc2)/3 |
| 1.731 and −1.199 | |
4. Conclusions
Supplementary Materials
Conflicts of Interest
References
- Hay, R.W.; Pujari, M.P. The preparation of cis-folded macrocyclic nickel(II) complexes and the kinetics of their base-catalysed conversion to planar species. J. Chem. Soc. Dalton Trans. 1986, 1485–1489. [Google Scholar]
- Black, D.G.; Jordan, R.F.; Rogers, R.D. Structural Trends in Group 4 Metal Tetraaza Macrocycle Complexes. Molecular Structures of (Me4taen)Zr(OtBu)2 and (Me4taen)Hf(NMe2)2. Inorg. Chem. 1997, 36, 103–108. [Google Scholar]
- Misra, T.K.; Chung, C.S.; Cheng, J.; Lu, T.H. Isolation and structural characterization of trans-β-{(C-meso-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)(diaquo)}nickel(II) perchlorate and cis-α-{(C-racemic-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane) (diisothiocyanato)}nickel(II). Polyhedron 2001, 20, 3149–3154. [Google Scholar]
- Curtis, N.F. Some cyclic tetra-amines and their metal-ion complexes. Part I. Two isomeric hexamethyltetra-azacyclotetradecanes and their copper(II) and nickel(II) complexes. J. Chem. Soc. 1964, 2644–2650. [Google Scholar]
- Warner, L.G.; Busch, D.H. Stereoisomers of a macrocyclic nickel(II) complex containing six asymmetric centers. Factors determining the stabilities of configurations and conformations. J. Am. Chem. Soc. 1969, 91, 4092–4101. [Google Scholar]
- Escuer, A.; Vicente, R.; Salah El Fallah, M.; Solans, X.; Font-Bardia, M. Synthesis, structure and magnetic properties of several nickel-azido complexes with tetraamines as blocking ligands. Inorg. Chim. Acta 1996, 247, 85–91. [Google Scholar]
- Escuer, A.; Vicente, R.; Ribas, J.; Salah J Fallah, M.; Solans, X. The first 1D nickel(II) complex with a single azido bridge: structure and magnetic behavior of catena-(μ-N3)[Ni(1,4,8,11-tetraazacyclotetradecane)](ClO4)•H2O. Inorg. Chem. 1993, 32, 1033–1035. [Google Scholar]
- Vicente, R.; Escuer, A.; Salah El Fallah, M.; Solans, X.; Font-Bardia, M. Three new mononuclear nickel(II) cyanate and isocyanate compounds derived from macrocyclic ligands: [Ni(TMCY) (NCO)](ClO4), [Ni(m-CTH)(OCN)1.5(ClO4)0.5]. Inorg. Chim. Acta 1997, 261, 227–237. [Google Scholar]
- Choi, K.Y.; Kim, Y.J.; Ryu, H.; Suh, I.H. Synthesis and characterization of nickel(II) complexes of a tetraaza macrocycle containing axial ligands. Inorg. Chem. Commun. 1999, 2, 176–180. [Google Scholar]
- Escuer, A.; Vicente, R.; Salah El Fallah, M.; Solans, X.; Font-Bardia, M. Structure and magnetic behaviour of the first singly bridged nickel cyanate chain and a new dinuclear complex: an approximation to the superexchange mechanism for the nickel pseudohalide system. J. Chem. Soc. Dalton Trans. 1996, 1013–1019. [Google Scholar] [CrossRef]
- Escuer, A.; Vicente, R.; Salah El Fallah, M.; Ribas, J.; Solans, X.; Font-Bardia, M. Unusual magnetic and structural one-dimensional nickel(II) azido systems. J. Chem. Soc. Dalton Trans. 1993, 2975–2976. [Google Scholar] [CrossRef]
- Hay, R.W.; Jeragh, B.; Ferguson, G.; Kaither, B.; Ruhl, B.L. Axial additions in nickel(II) complexes of (5SR,7RS,12RS,14SR)-tetra-methyl-1,4,8,11-tetra-azacyclotetradecane (Lα) and crystal-structure analysis of the planar orange and octahedral violet perchlorate salts NiLα,(ClO4)2. J. Chem. Soc. Dalton Trans. 1982, 1531–1539. [Google Scholar] [CrossRef]
- Hay, R.W.; Danby, A.; Lighfoot, P.; Lampeka, Y.D. Padlock macrocyclic complexes. The synthesis of a range of nickel(II) complexes of N-alkyl azacyclams and the crystal structure of (3-ethyl-1,3,5,8,12-penta-azacyclotetradecane) nickel(II) perchlorate. Polyhedron 1997, 16, 2777–2783. [Google Scholar] [CrossRef]
- Hay, R.W.; Crayston, J.A.; Cromie, T.J.; Lighfoot, P.; de Alwis, D.C.L. The preparation, chemistry and crystal structure of the nickel(II) complex of N-hydroxyethylazacyclam [3-(2′-hydoxyethyl)-1,3,5,8,12-pentaazacyclotetradecane nickel(II) perchlorate]. A new electrocatalyst for CO2 reduction. Polyhedron 1997, 16, 3557–3563. [Google Scholar] [CrossRef]
- Choi, K.Y.; Kim, J.C.; Jensen, W.P.; Suh, I.H.; Choi, S.-S. Copper(II) and Nickel(II) Complexes with 3,14-Dimethyl-2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosane, [M(C20H40N4)]Cl2 • 2H2O (M = CuII and NiII). Acta Crystallogr. 1996, C52, 2166–2168. [Google Scholar]
- Curtis, N.F.; Wikaira, J. Nickel(II) compounds of a tri-amine mono-imine macrocycle: Preparations and structures of (5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradec-4-ene)nickel(II) compounds. Polyhedron 2011, 30, 895–902. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chun, K.M.; Suh, I.H. Synthesis and characterization of one-dimensional nickel(II) macrocyclic complexes with bridging organic ligands. Polyhedron 2001, 20, 57–65. [Google Scholar] [CrossRef]
- Choi, K.Y.; Kim, K.J. Self-assembly of 1D coordination polymers from nickel(II) tetraaza macrocycle and carboxylate ligands. Polyhedron 2008, 27, 1310–1316. [Google Scholar] [CrossRef]
- Fenton, D.D.; Okawa, H. Dalton perspective. The emergence of trinuclear constellations at metallobiosites. J. Chem. Soc. Dalton Trans. 1993, 1349–1357. [Google Scholar] [CrossRef]
- Tsukube, H.; Yoden, T.; Iwachido, T.; Zenki, M. Lipid-bound macrocycles as new immobilized ligand systems for effective separation of metal cations. J. Chem. Soc. Chem. Commun. 1991, 1069–1070. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows - a version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Bakalbassis, E.G.; Tsipis, C.A.; Bozopoulos, A.P.; Dreissing, D.W.; Hartl, H.; Mrozinski, J. Strong ferromagnetism between copper(II) ions separated by 6.7.ANG. in a new phthalato-bridged copper(II) binuclear complex. Inorg. Chem. 1991, 30, 2801–2806. [Google Scholar]
- Cao, R.; Shi, Q.; Sun, D.; Hong, M.; Bi, W.; Zhao, Y. Syntheses and characterizations of copper(II) polymeric complexes constructed from 1,2,4,5-benzenetetracarboxylic acid. Inorg. Chem. 2002, 41, 6161–6168. [Google Scholar] [CrossRef] [PubMed]
- Vigee, G.S.; Watkins, C.L.; Bowen, H.F. The chemical dynamics of 1,4,8,11-tetraazacyclotetradecane nickel(II) perchlorate with several solvents. I. Thermodynamics of base adduct formation. Inorg. Chim. Acta 1979, 35, 255–259. [Google Scholar] [CrossRef]
- North, A.C.T.; Phillips, D.C.; Mathews, F.S. A semi-empirical method of absorption correction. Acta Crystallogr. 1968, A24, 351–359. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the [Ni(L)(isoph)2][Ni(L)]·8H2O is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, I.-T.; Choi, K.-Y. Self-Assembly of a 1D Hydrogen-Bonded Polymer from a Hexamethyltetraaza Macrocyclic Nickel(II) Complex and Isophthalic Acid. Molecules 2013, 18, 6608-6619. https://doi.org/10.3390/molecules18066608
Lim I-T, Choi K-Y. Self-Assembly of a 1D Hydrogen-Bonded Polymer from a Hexamethyltetraaza Macrocyclic Nickel(II) Complex and Isophthalic Acid. Molecules. 2013; 18(6):6608-6619. https://doi.org/10.3390/molecules18066608
Chicago/Turabian StyleLim, In-Taek, and Ki-Young Choi. 2013. "Self-Assembly of a 1D Hydrogen-Bonded Polymer from a Hexamethyltetraaza Macrocyclic Nickel(II) Complex and Isophthalic Acid" Molecules 18, no. 6: 6608-6619. https://doi.org/10.3390/molecules18066608




