Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor
Abstract
:1. Introduction
2. Flavor Measurement
2.1. Sensory Analysis
2.2. Instrumental Flavor Analysis
3. Relating Sensory and Instrumental Methods—Why and How
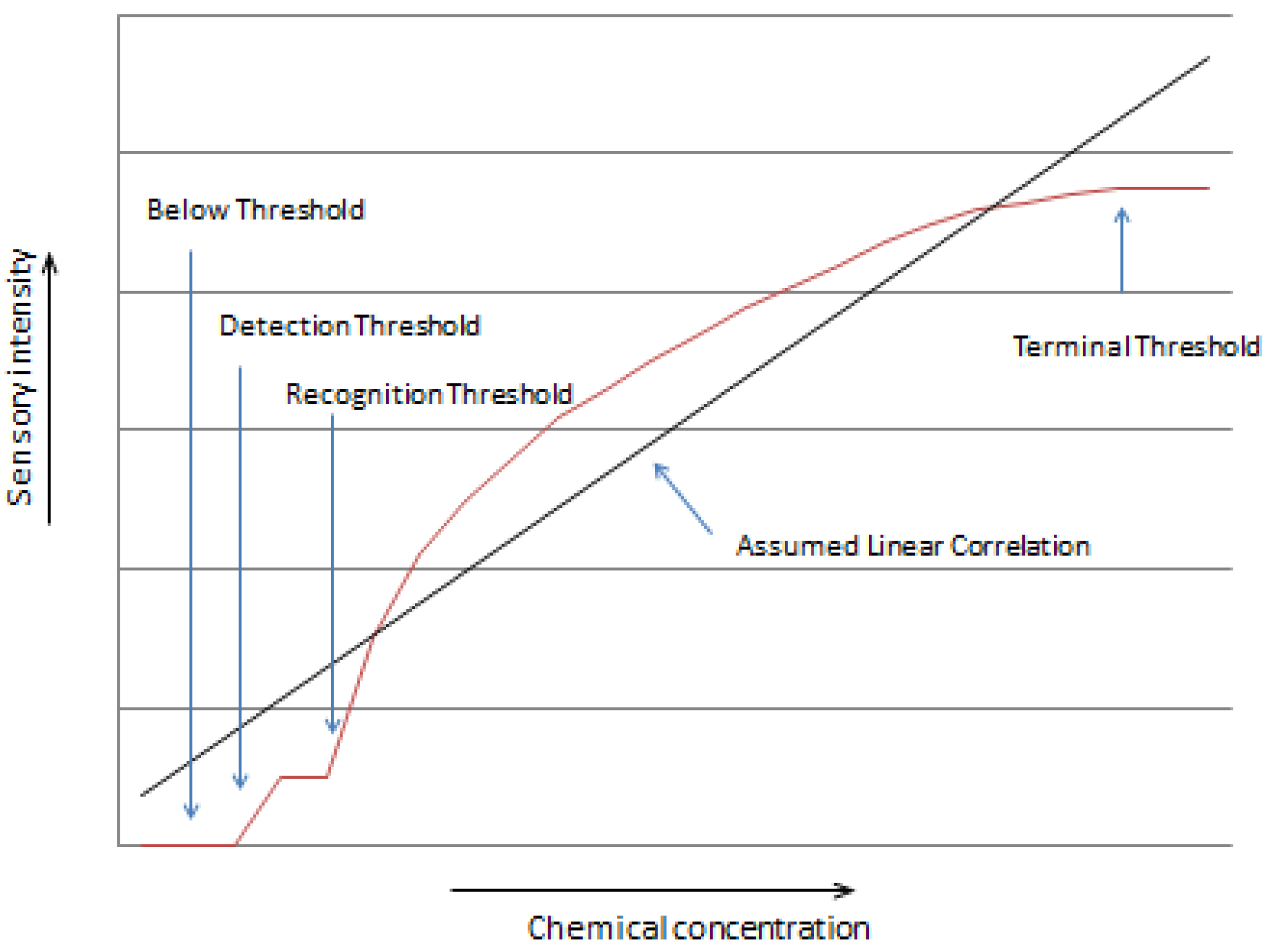
3.1. Direct Relationships
3.2. Calculated Relationships: Correlation and Regression
4. Examples of Identified Relationships and Potential Problems
4.1. Hexanal
4.2. 3-Methyl-1-butanol
5. Issues in Identifying Relationships
5.1. Poor Measurement and Identification
5.2. “Noise” from Other Compounds and Attributes
5.3. Overlap and Variable Naming of Sensory Terminology
5.4. Unexpected Relationships
5.5. Statistical Issues
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Foods: Principles and Practices, 2nd ed; Chapman & Hall: New York, NY, USA, 2010; pp. 1–18, 125–148. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Advanced Statistical Methods. In Sensory Evaluation Techniques, 4th ed; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Verriele, M.; Plaisance, H.; Vandenbilcke, V.; Locoge, N.; Jaubert, J.N.; Meunier, G. Odor evaluation and discrimination of car cabin and its components: Application of the “field of odors” approach in a sensory descriptive analysis. J. Sens. Stud. 2012, 27, 103–110. [Google Scholar]
- Maarse, H. Itroduction. In Volatile Compounds in Foods and Beverages, 1st; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 1–39. [Google Scholar]
- Dreyer, E.; Sims, C.; Rousseff, R.; Gray, D.; Sipowicz, M. Sensory and compositional characteristics of Blanc Du Bois wine. Am. J. Enol. Vitic. 2013, 64, 118–125. [Google Scholar] [CrossRef]
- Gamero, A.; Wesselink, W.; de Jong, C. Comparison of the sensitivity of different aroma extraction techniques in combination with gas chromatography-mass spectrometry to detect minor aroma compounds in wine. J. Chromatogr. A 2013, 1272, 1–7. [Google Scholar] [CrossRef]
- Saenz-Navajas, M.P.; Fernandez-Zurbano, P.; Ferreira, V. Contribution of nonvolatile composition to wine flavor. Food Res. Int. 2012, 28, 389–411. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Deng, J.; Li, H.; Lu, J. Phenolic concentrations and antioxidant properties of wines made from North American grapes grown in China. Molecules 2012, 17, 3304–3323. [Google Scholar] [CrossRef]
- Sanchez, G.; Besada, C.; Badenes, M.S.; Monforte, A.J.; Granell, A. A non-targeted approach unravels the volatile network in peach fruit. PLoS One 2012, 7, e38992. [Google Scholar]
- Wei, C.-B.; Liu, S.-H.; Liu, Y.-G.; Lv, L.-L.; Yang, W.-X.; Sun, G.-M. Characteristic aroma compounds from different pineapple parts. Molecules 2011, 16, 5104–5112. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sanudo, C.; Escudero, A. Gas chromatographic-olfactometric aroma profile and quantitative analysis of volatile carbonyls of grilled beef from different finishing feed systems. J. Food Sci. 2012, 77, S240–S246. [Google Scholar] [CrossRef]
- Varming, C.; Andersen, L.T.; Petersen, M.A.; Ardo, Y. Flavour compounds and sensory characteristics of cheese powders made from matured cheeses. Int. Dairy J. 2013, 30, 19–28. [Google Scholar] [CrossRef]
- Mohamed, H.N.; Man, Y.C.; Mustafa, S.; Manap, Y.A. Tentative identification of volatile flavor compounds in commercial budu, a Malaysian fish sauce, using GC-MS. Molecules 2012, 17, 5062–5080. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, J.; Peng, L.; Zou, L.; Wang, J.; Zhong, L.; Xiang, D. Effects of yeast polysaccharide on growth and flavonoid accumulation in Fagopyrum tataricum sprout cultures. Molecules 2012, 17, 11335–11345. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; Lopez, M.L.; Echeverria, G. Relationships between the instrumental and sensory characteristics of four peach and nectarine cultivars stored under air and CA atmospheres. Postharvest Biol. Technol. 2013, 75, 58–67. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J.C. Polyphenols, antioxidant potential and color of fortified wines during accelerated ageing: The madeira wine case study. Molecules 2013, 18, 2997–3017. [Google Scholar] [CrossRef]
- de Beer, D.; Schulze, A.E.; Joubert, E.; de Villiers, A.; Malherbe, C.J.; Stander, M.A. Food ingredient extracts of Cyclopiasubternata (Honeybush): Variation in phenolic composition and antioxidant capacity. Molecules 2012, 17, 14602–14624. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Li, J.M.; Zhang, B.C.; Yu, Y.; Shen, C.H.; Song, P. A comparison of the influence of eight commercial yeast strains on the chemical and sensory profiles of freshly distilled Chinese brandy. J. Inst. Brewing 2012, 118, 315–324. [Google Scholar] [CrossRef]
- Poinot, P.; Arvisenet, G.; Ledauphin, J.; Gaillard, J.L.; Prost, C. How can aroma-related cross-modal interactions be analysed? A review of current methodologies. Food Qual. Prefer. 2013, 28, 304–316. [Google Scholar] [CrossRef]
- Ross, C.F. Sensory science at the human-machine interface. Trends Food Sci. Tech. 2009, 20, 63–72. [Google Scholar] [CrossRef]
- Croissant, A.E.; Watson, D.M.; Drake, M.A. Application of sensory and instrumental analyses to dairy products. Annu. Rev. Food Sci. Technol. 2009, 2, 395–421. [Google Scholar] [CrossRef]
- Auvray, M.; Spence, C. The multisensory perception of flavor. Consciousness Cogn. 2008, 17, 1016–1031. [Google Scholar] [CrossRef]
- Hootman, R.C. ASTM Manual on Descriptive Analysis Testing for Sensory Evaluation; American Society for Testing and Materials: Philadelphia, PA, USA, 1992; pp. 1–3. [Google Scholar]
- Chambers, D.H.; Allison, A.A.; Chambers, E., IV. Effects of Training on Performance of Descriptive Sensory Panelists. J. Sens. Stud. 2004, 19, 486–499. [Google Scholar] [CrossRef]
- Maughan, C.; Martini, S. Identification and quantification of flavor attributes present in chicken, lamb, pork, beef, and turkey. J. Food Sci. 2012, 77, S115–S121. [Google Scholar] [CrossRef]
- Adhikari, K.; Chambers, E., IV; Miller, R.; Vázquez-Araújo, L.; Bhumiratana, N.; Philip, C. Development of a lexicon for beef flavor in intact muscle. J. Sens. Stud. 2011, 26, 413–420. [Google Scholar] [CrossRef]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the sensory description of Australian native plant foods and ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Chambers, E., IV; Lee, J.; Chun, S.; Miller, A. Development of a lexicon for commercially available cabbage (baechu) kimchi. J. Sens. Stud. 2012, 27, 511–518. [Google Scholar] [CrossRef]
- Suwonsichon, S.; Chambers, E., IV; Kongpensook, V.; Oupadissakoon, C. Sensory lexicon for mango as affected by cultivars and stages of ripeness. J. Sens. Stud. 2012, 27, 148–160. [Google Scholar]
- Talavera-Bianchi, M.; Chambers, E., IV; Chambers, D.H. Lexicon to describe flavor of fresh leafy vegetables. J. Sens. Stud. 2010, 25, 163–183. [Google Scholar] [CrossRef]
- Miller, A.E.; Chambers, E., IV; Jenkins, A.; Lee, J.; Chambers, D.H. Defining and characterizing the “Nutty” attribute across food categories. Food Qual. Prefer. 2013, 27, 1–7. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Chambers, D.; Carbonell-Barrachina, A.A. Development of a sensory lexicon and application by an industry trade panel for turrón, a European protected product. J. Sens. Stud. 2012, 27, 26–36. [Google Scholar] [CrossRef]
- Civille, G.V.; Lapsley, K.; Huang, G.; Yada, S.; Seltsam, J. Development of an almond lexicon to assess the sensory properties of almond varieties. J. Sens. Stud. 2010, 25, 146–162. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Lopetcharat, K.; Guthrie, B.; Drake, M.A. Descriptive analysis of carbonated regular and diet lemon-lime beverages. J. Sens. Stud. 2012, 25, 247–263. [Google Scholar]
- Bett-Garber, K.L.; Lea, J.M.; Champagne, E.T.; McClung, A.M. Whole-grain rice flavor associated with assorted bran colors. J. Sens. Stud. 2012, 27, 78–86. [Google Scholar] [CrossRef]
- Elía, M. A procedure for sensory evaluation of bread: protocol developed by a trained panel. J. Sens. Stud. 2011, 26, 269–277. [Google Scholar] [CrossRef]
- Callejo, M.J. Present situation on the descriptive sensory analysis of bread. J. Sens. Stud. 2011, 26, 255–268. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Di Donfrancesco, B.; Koppel, K.; Chambers, E., IV. An initial lexicon for sensory properties of dry dog food. J. Sens. Stud. 2012, 27, 498–510. [Google Scholar]
- Poehlmann, S.; Schieberle, P. Characterization of the aroma signature of Styrian pumpkin seed oil (Cucurbitapepo subsp. pepo var. Styriaca) by molecular sensory science. J. Agric. Food Chem. 2013, 61, 2933–2942. [Google Scholar] [CrossRef]
- Careri, M.; Bianchi, F.; Corradini, C. Recent advances in the application of mass spectrometry in food-related analysis. J. Chromatogr. A 2002, 970, 3–64. [Google Scholar] [CrossRef]
- Johnson, A.J.; Hirson, G.D.; Ebeler, S.E. Perceptual characterization and analysis of aromamixtures using gas chromatography recomposition-olfactometry. PLoS One 2012, 7, e42693. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.; Sims, C.; Baldwin, E.; Balaban, M. Tomato flavor and aroma quality as affected by storage temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Berna, A.; Buysens, S.; Di Natale, C.; Grun, I.; Lammertyn, J. Relating sensory analysis with electronic nose and headspace fingerprint MS for tomato aroma profiling. Postharvest Biol. Technol. 2005, 36, 143–155. [Google Scholar] [CrossRef]
- Dymerski, T.M.; Chmiel, T.M.; Wardencki, W. An odor-sensing-system—Powerful technique for foodstuff studies. Rev. Sci. Instrum. 2011, 82, 1–32. [Google Scholar]
- Tesfaye, W.; Morales, M.L.; Garcia-Parrilla, M.C.; Troncoso, A.M. Improvement of wine vinegar elaboration and quality analysis: Instrumental and human sensory evaluation. Food Rev. Int. 2009, 25, 142–156. [Google Scholar] [CrossRef]
- Smith, G.L. Statistical analysis of sensory data. In Sensory Analysis of Foods, 2nd; Piggott, J.R., Ed.; Elsevier Applied Science: New York, NY, USA, 1988; pp. 335–379. [Google Scholar]
- Hongsoongnern, P.; Chambers, E. A lexicon for green odor or flavor and characteristics of chemicals associated with green. J. Sens. Stud. 2008, 23, 205–221. [Google Scholar] [CrossRef]
- Vara-Ubol, S.; Chambers, E.; Chambers, D.H. Sensory characteristics of chemical compounds potentially associated with beany aroma in foods. J. Sens. Stud. 2004, 19, 15–26. [Google Scholar]
- Bott, L.; Chambers, E. Sensory characteristics of combinations of chemicals potentially associated with beany aroma in foods. J. Sens. Stud. 2006, 21, 308–321. [Google Scholar]
- Martens, M.; Martens, H.; Wold, S. Preference of cauliflower related to sensory descriptive variables by partial least squares (PLS) regression. J. Sci. Food Agric. 1983, 34, 715–724. [Google Scholar] [CrossRef]
- Lee, J.; Vazquez-Araujo, L.; Adhikari, K.; Warmund, M.; Elmore, J. Volatile compounds in light, medium, and dark black walnut and their influence on the sensory aromatic profile. J. Food Sci. 2011, 76, C199–C204. [Google Scholar] [CrossRef]
- Koppel, K.; Adhikari, K.; Di Donfrancesco, B. Volatile compounds in dry dog foods and their influence on sensory aromatic profile. Molecules 2013, 18, 2646–2662. [Google Scholar]
- Yenket, R.; Chambers, E., IV; Adhikari, K. A comparison of seven preference mapping techniques using four software programs. J. Sens. Stud. 2011, 26, 135–150. [Google Scholar]
- Abegaz, E.G.; Tandon, K.S.; Scott, J.W.; Baldwin, E.A.; Shewfelt, R.L. Partitioning taste from aromatic flavor notes of fresh tomato (Lycopersicon esculentum, Mill) to develop predictive models as a function of volatile and nonvolatile components. Postharvest Biol. Technol. 2004, 34, 224–235. [Google Scholar]
- Baldwin, E.A.; Goodner, K.; Plotto, A.; Pritchett, K.; Einstein, M. Effect of volatiles and their concentration on perception of tomato descriptors. J. Food Sci. 2004, 69, 310–318. [Google Scholar] [CrossRef]
- Du, X.F.; Kurnianta, A.; McDaniel, M.; Finn, C.E.; Qian, M.C. Flavourprofiling of “Marion” and thornless blackberries by instrumental and sensory analysis. Food Chem. 2010, 121, 1080–1088. [Google Scholar] [CrossRef]
- Lozano, P.R.; Drake, M.; Benitez, D.; Cadwallader, K.R. Instrumental and sensory characterization of heat-induced odorants in aseptically packaged soy milk. J. Agric. Food Chem. 2007, 55, 3018–3026. [Google Scholar] [CrossRef]
- Miyazaki, T.; Plotto, A.; Baldwin, E.A.; Reyes-De-Corcuera, J.; Gmitter, F.G. Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J. Sci. Food Agric. 2012, 92, 727–735. [Google Scholar] [CrossRef]
- Carunchia-Whetstine, M.E.; Drake, M.A.; Nelson, B.K.; Barbano, D.M. Flavor profiles of full-fat and reduced-fat cheese and cheese fat made from aged cheddar with the fat removed using a novel process. J. Dairy Sci. 2006, 89, 505–517. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Cultivar influence on virgin olive (Olea europea L.) oil flavor based on aromatic compounds and sensorial profile. Sci. Hortic. 2008, 118, 139–148. [Google Scholar]
- Schulbach, K.; Rouseff, R.; Sims, C. Relating descriptive sensory analysis to gas chromatography/olfactometry ratings of fresh strawberries using partial least squares regression. J. Food Sci. 2004, 69, S273–S277. [Google Scholar] [CrossRef]
- Tandon, K.S. Linking sensory descriptors to volatile and nonvolatile components of fresh tomato flavor. J. Food Sci. 2003, 68, 2366–2371. [Google Scholar] [CrossRef]
- Alasalvar, C.; Topal, B.; Serpen, A.; Bahar, B.; Pelvan, E.; Gökmen, V. Flavor characteristics of seven grades of black tea produced in Turkey. J. Agric. Food Chem. 2012, 60, 6323–6332. [Google Scholar] [CrossRef]
- Alasalvar, C.; Odabasi, A.Z.; Demir, N.; Balaban, M.Ö.; Shahidi, F.; Cadwallader, K.R. Volatiles and flavor of five Turkish hazelnut varieties as evaluated by descriptive sensory analysis, electronic nose, and dynamic headspace analysis/gas-chromatography-mass spectrometry. J. Food Sci. 2004, 69, 99–106. [Google Scholar]
- Alasalvar, C.; Shahidi, F.; Cadwallader, K.R. Comparison of natural and roasted Turkish tombul hazelnut (Corylus avellana L.) volatiles and flavor by DHA/GC/MS and descriptive sensory analysis. J. Agric. Food Chem. 2003, 51, 5067–5072. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, N.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar]
- Bubola, K.B.; Koprivnjak, O.; Sladonja, B.; Lukić, I. Volatile compounds and sensory profiles of monovarietal virgin olive oil from Buža, Črna, and Rosinjola cultivars in Istria (Croatia). Food Technol. Biotech. 2012, 50, 192–198. [Google Scholar]
- Bach, V.; Kidmose, U.; Bjørn, G.K.; Edelenbos, M. Effects of harvest time and variety on sensory quality and chemical composition of Jerusalem artichoke (Helianthus tuberosus) tubers. Food Chem. 2012, 133, 82–89. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-Olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Elmaci, Y.; Onogur, T.A. Mandarin peel aroma: estimation by using headspace/GC/MS and descriptive analysis techniques. Acta Aliment. 2012, 41, 131–139. [Google Scholar] [CrossRef]
- Frank, D.; Poole, S.; Kircchoff, S.; Forde, C. Investigation of sensory and volatile characteristics of farmed and wild barramundi (Latescalcarifer) using gas chromatography-olfactometry mass spectrometry and descriptive sensory analysis. J. Agric. Food Chem. 2009, 57, 10302–10312. [Google Scholar] [CrossRef]
- Karahadian, C.; Johnson, K. Analysis of headspace volatiles and sensory characteristics of fresh corn tortillas made from fresh masa dough and spray-dried masa flour. J. Agric. Food Chem. 1993, 41, 791–799. [Google Scholar] [CrossRef]
- Lopez-Nicolas, J.M.; Andreu-Sevilla, A.J.; Carbonell-Barrachina, A.A.; Garcia Carmona, F. Effects of addition of Alpha-Cyclodextrin on the sensory quality, volatile compounds, and color parameters of fresh pear juice. J. Agric. Food Chem. 2009, 57, 9668–9675. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Diaz-Maroto, M.C.; Gonzalez-Vinas, M.A.; Perez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Ercan, D.; Korel, F.; Yüceer, K.Y.; Kınık, Ö. Physicochemical, textural, volatile, and sensory profiles of traditional sepet cheeses. J. Dairy Sci. 2011, 94, 4300–4312. [Google Scholar] [CrossRef]
- Flores, M.; Grimm, C.C.; Toldrá, F.; Spanier, A.M. Correlations of sensory and volatile compounds of Spanish “Serrano” dry-cured ham as a function of two processing times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Forde, C.; Cox, A.; Williams, E.; Boss, P. Associations between the sensory attributes and volatile composition of cabernet sauvignon wines and the volatile composition of the grapes used for their production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar]
- Krumbein, A.; Peters, P.; Bruckner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Limpawattana, M.; Yang, D.S.; Kays, S.J.; Shewfelt, R.L. Relating sensory descriptors to volatile components in flavor of specialty rice types. J. Food Sci. 2008, 73, S456–S461. [Google Scholar] [CrossRef]
- Mitchell, M.; Brunton, N.P.; Wilkinson, M.G. Impact of salt reduction on the instrumental and sensory flavor profile of vegetable soup. Food Res. Int. 2011, 44, 1036–1043. [Google Scholar] [CrossRef]
- Whitson, M.E.; Miracle, R.E.; Drake, M.A. Sensory characterization of chemical components responsible for cardboard flavor in whey protein. J. Sens. Stud. 2010, 25, 616–636. [Google Scholar]
- Yoder, W.M.; Currlin, S.W.; Larue, A.; Fernandex, K.M.; King, D.; Smith, D.W. Interactions of guaiacol and methyl salicylate in binary mixture significantly lowers perceptual threshold in human observers. J. Sens. Stud. 2012, 27, 161–167. [Google Scholar]
- Saenz-Navajas, M.; Campo, E.; Avizcuri, J.M.; Valentin, D.; Fernandez-Zurbano, P.; Ferreira, V. Contribution of non-volatile and aroma fractions to in-mouth sensory properties of red wines: Wine reconstitution strategies and sensory sorting task. Anal. Chim. Acta 2012, 732, 64–72. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef]
- Heil, M.; Hahn, H.; Christoph, N.; Stingel, D.; Gessler, A.; Ara, V. 3-Methylbutanol in apple juice. Fruit Process. 2007, 17, 164–176. [Google Scholar]
- Costello, P.J.; Francis, I.L.; Bartowsky, E.J. Variations in the effect of malolactic fermentation on the chemical and sensory properties of cabernet sauvignon wine: Interactive influences of oenococcusoeni strain and wine matrix composition. Aust. J. Grape. Wine R 2012, 18, 287–301. [Google Scholar] [CrossRef]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Torrens, J.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Assessment of volatile and sensory profiles between base and sparkling wines. J. Agric. Food Chem. 2010, 58, 2455–2461. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Sampaio, P. Volatile fraction of DOP “CasteloBranco” cheese: Influence of breed. Food Chem. 2009, 112, 1053–1059. [Google Scholar] [CrossRef]
- Moio, L.; Dekimpe, J.; Etievant, P.X.; Addeo, F. Volatile flavour compounds of water buffalo mozzarella cheese. Italian J. Food Sci. 1993, 1, 57–68. [Google Scholar]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y. Identification of distinctive volatile compounds in fish sauce. J. Agric. Food Chem. 2002, 50, 5412–5416. [Google Scholar]
- Garcia-Carpintero, G.E.; Gomez-Gallego, M.A.; Sanchez-Palomo, E.; Gonzalez-Vinas, M.A. Impact of alternative technique to ageing using oak chips in alcoholic or in malolactic fermentation on volatile and sensory composition of red wines. Food Chem. 2012, 134, 851–863. [Google Scholar]
- Garcia-Gonzales, D.L.; Tena, N.; Aparicio, R. Characterization of olive paste volatiles to predict the sensory quality of virgin olive oil. Eur. J. Lipid. Sci. Technol. 2007, 109, 663–672. [Google Scholar] [CrossRef]
- Aznar, M.; Lopez, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef]
- Bendini, A.; Barbieri, S.; Valli, E.; Buchecker, K.; Canavari, M.; Toschi, T.G. Quality evaluation of cold pressed sunflower oils be sensory and chemical analysis. Eur. J. Lipid Sci. Technol. 2011, 113, 1375–1384. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, S.; Koh, K. Development of korean red wines using Vitis labrusca Varieties: Instrumental and sensory characterization. Food Chem. 2006, 94, 385–393. [Google Scholar] [CrossRef]
- Niu, Y. Characterization of odor-active compounds of various cherry wines by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. J. Chromatogr. B 2011, 879, 2287–2293. [Google Scholar] [CrossRef]
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Vilanova, M.; Campo, E.; Escudero, A.; Grana, M.; Masa, A. Volatile composition and sensory properties of vitis vinifera red cultivars from north-west Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Jonsdottir, R.; Olafsdottir, G.; Martinsdottir, E.; Stefansson, G. Flavor characterization of ripened cod roe by gas chromatography, sensory analysis, and electronic nose. J. Agric. Food Chem. 2004, 52, 6250–6256. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Bendini, A.; Tesini, F.; Valli, E.; Lamuela-raventos, R.M.; Toschi, T.G. Chemical and sensory analysis of commercial tomato juices present on the Italian and Spanish markets. J. Agric. Food Chem. 2013, 61, 1044–1050. [Google Scholar] [CrossRef]
- Hansen, Å.; Lund, B.; Lewis, M.J. Flavour of sourdough rye bread crumb. Z. Lebensm.-Unters. Forsch. 1989, 22, 141–144. [Google Scholar]
- Ruth, S.M.; O’Connor, C.H. Evaluation of three gas chromatography-olfactometry methods: comparison of odour intensity-concentration relationships of eight volatile compounds with sensory headspace data. Food Chem. 2001, 74, 341–347. [Google Scholar] [CrossRef]
- Otremba, M.; Dikeman, M.E.; Milliken, G.A.; Stroda, S.L.; Chambers, E., IV; Chambers, D.H. Interrelationships between a descriptive texture profile sensory panel and descriptive attribute sensory panel evaluations of Longissimus and Semitendinosus muscles. Meat Sci. 2000, 54, 325–332. [Google Scholar] [CrossRef]
- Ito, Y.; Kubota, K. Sensory evaluation of the synergism among odorants present in concentrations below their odor threshold in a Chinese jasmine green tea infusion. Mol. Nutr. Food Res. 2005, 49, 61–68. [Google Scholar] [CrossRef]
- Dalton, P.; Doolittle, N.; Nagata, H.; Breslin, P.A.S. The merging of the senses: integration of subthreshold taste and smell. Nat. Neurosci. 2000, 3, 431–432. [Google Scholar] [CrossRef]
- Donishi, T.; Kimura, A.; Imbe, H.; Yokoi, I.; Kaneoke, Y. Sub-threshold cross-modal sensory interaction in the tahalamus: Lemniscal auditory response in the medial geniculate nucleus is modulated by somatosensory stimulation. Neuroscience 2011, 174, 200–215. [Google Scholar] [CrossRef]
- Wilson, C.E.; Brown, W.E. Influence of food matrix structure and oral breakdown during mastication on temporal perception of flavor. J. Sens. Stud. 1997, 12, 69–86. [Google Scholar] [CrossRef]
- Salles, C.; Chagnon, M.C.; Feron, G.; Guichard, E.; Laboure, H.; Morzel, M.; Semon, E.; Tarrega, A.; Yven, C. In-mouth mechanisms leading to flavor release and perception. Crit. Rev. Food Sci. 2001, 51, 67–90. [Google Scholar]
- Voilley, A.; Lubbers, S. Flavor-matrix interactions in wine. In Chemistry of Wine Flavor; Waterhouse, A.L., Ebeler, S.E., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 217–229. [Google Scholar]
- Guichard, E. Interactions between flavor compunds and food ingredients and their influence on flavor perception. Food Rev. Intern. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Osborne, J.W. Logits and tigers and bears, oh my! A brief look at the simple math of logistic regression and how it can improve dissemination of results. Pract. Assess. Res. Eval. 2012, 17, 1–10. [Google Scholar]
- Osborne, J.W.; Overbay, A. The power of outliers (and why researchers should always check for them). Pract. Assess. Res. Eval. 2004, 9, 6. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chambers, E., IV; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887-4905. https://doi.org/10.3390/molecules18054887
Chambers E IV, Koppel K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules. 2013; 18(5):4887-4905. https://doi.org/10.3390/molecules18054887
Chicago/Turabian StyleChambers, Edgar, IV, and Kadri Koppel. 2013. "Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor" Molecules 18, no. 5: 4887-4905. https://doi.org/10.3390/molecules18054887





