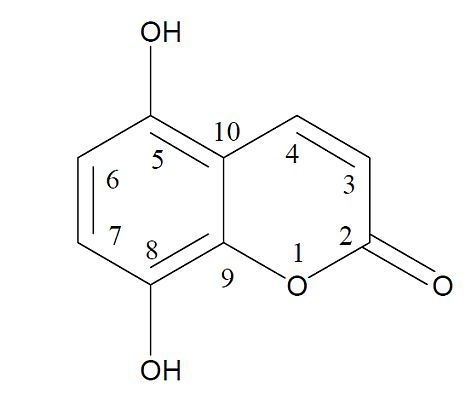
Evaluation of the Biological Activity of Naturally Occurring 5,8-Dihydroxycoumarin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Properties of 5,8-Dihydroxycoumarin

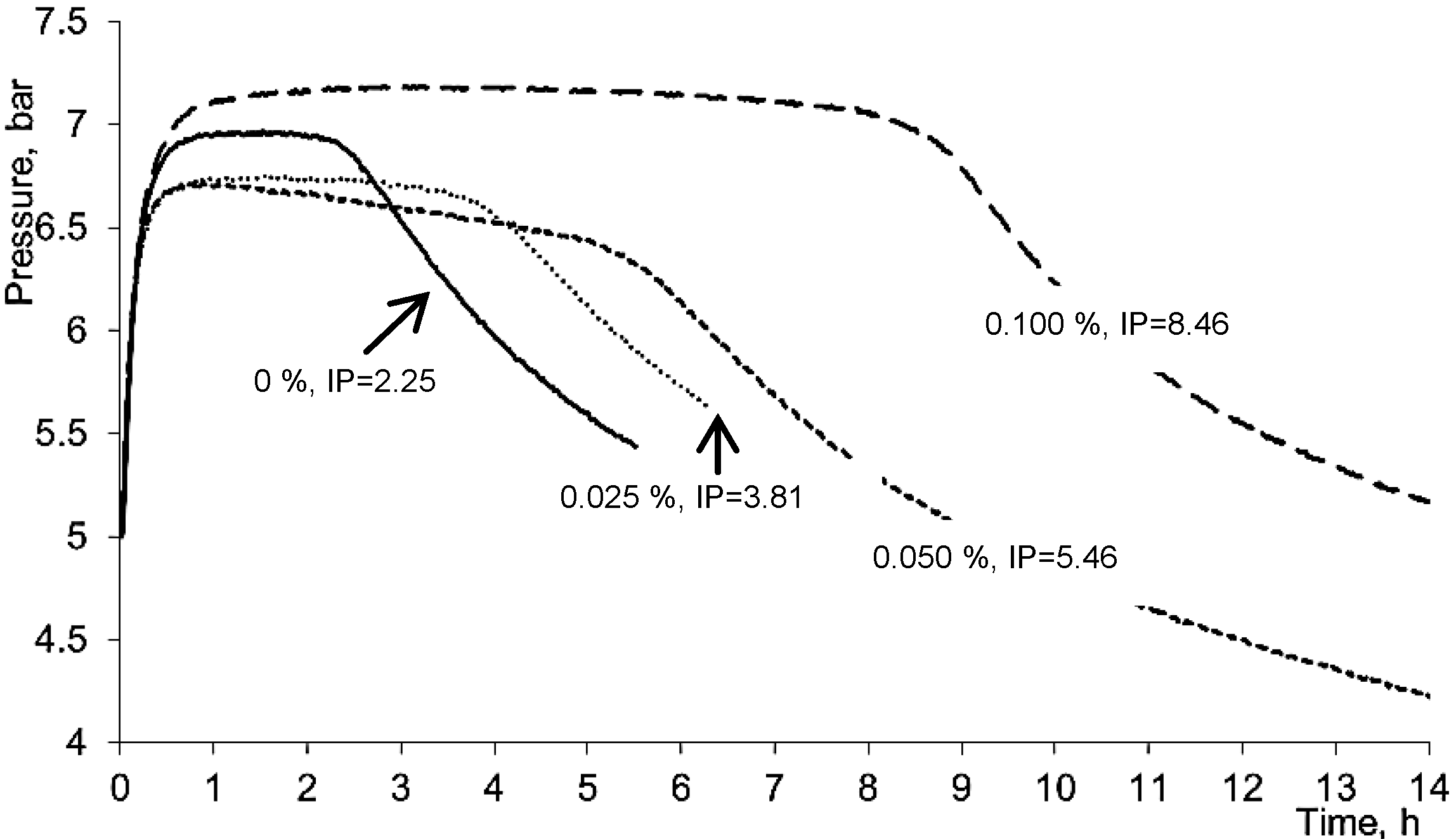
2.2. Genotoxic Potential of 5,8-Dihydroxycoumarin
2.2.1. Induction of Chromosome Aberrations and Micronuclei in Rat Bone Marrow Cells in Vivo
| Treatment group | Sacrifice time (h) | Dose (mg/kg b.w.) | Aberrant metaphases (%± S.E.M.) | MNPCE (%± S.E.M.) | PCE (%± S.E.M.) |
|---|---|---|---|---|---|
| Blank control | 24 | 0 | 1.50 ± 0.27 | 0.21 ± 0.04 | 36.4 ± 5.5 |
| Single intraperitoneal treatment | |||||
| CP (positive control) | 24 | 30 | 21.83 ± 3.25 a | 1.67 ± 0.22 a | 34.8 ± 6.4 |
| Vehicle | 24 | 1.33 ± 0.21 | 0.38 ± 0.04 | 30.1 ± 3.4 | |
| 48 | 2.00 ± 0.36 | 0.25 ± 0.03 | 36.0 ± 4.4 | ||
| 5,8-DHC | 24 | 10 | 2.33 ± 0.49 | 0.56 ± 0.08 a | 32.1 ± 1.9 |
| 48 | 10 | 1.67 ± 0.56 | 0.30 ± 0.06 | 31.8 ± 5.6 | |
| 24 | 20 | 1.00 ± 0.36 | 0.63 ± 0.04 a | 36.6 ± 2.8 | |
| 48 | 20 | 2.60 ± 0.68 | 0.59 ± 0.14a | 26.6 ± 5.0 | |
| Repeated treatment via gavage | |||||
| Vehicle | 48 | 1.67 ± 0.21 | 0.24 ± 0.04 | 48.8 ± 5.1 | |
| 5,8-DHC | 48 | 3 × 10 | 1.17 ± 0.17 | 0.24 ± 0.05 | 31.7 ± 4.0 |
| 48 | 3 × 20 | 2.43 ± 0.37 | 0.23 ± 0.05 | 45.5 ± 3.5 | |
2.2.2. Induction of Chromosome Aberrations and SCEs in Human Lymphocytes in Vitro
| Treatment | Concentration (µg/mL) | CA per 100 cells a (±S.E.M.) | Donor A | Donor B | ||
|---|---|---|---|---|---|---|
| SCE/
cell ± S.E.M. | RI ± S.E.M | SCE/
cell ± S.E.M. | RI ± S.E.M | |||
| Blank | 1.5 ± 0.9 | 9.9 ± 0.5 | 2.72 ± 0.04 | 8.7 ± 0.4 | 2.45 ± 0.05 | |
| Ethanol | 7.5 µL/mL | 3.0 ± 1.2 | 10.6 ± 0.4 | 2.76 ± 0.04 | 9.5 ± 0.5 | 2.46 ± 0.05 |
| MMS | 0.02 µL/mL | 12.0 ± 3.2 b | 31.7 ± 1.2 b | 2.21 ± 0.05 b | 46.7 ± 1.9 b | 2.10 ± 0.06 b |
| 5,8-DHC | 10 | 1.5 ± 0.9 | 10.2 ± 0.5 | 2.70 ± 0.04 | 10.1 ± 0.5 | 2.22 ± 0.05 b |
| 15 | 3.0 ± 1.2 | 12.3 ± 0.6 b | 2.74 ± 0.31 | 10.6 ± 0.5 | 2.35 ± 0.05 | |
| 20 | 3.5 ± 1.3 | 12.7 ± 0.7 b | 2.65 ± 0.04 b | 10.3 ± 0.4 | 2.49 ± 0.05 | |
| 25 | 6.5 ± 1.7 | 13.6 ± 0.6 b | 2.66 ± 0.04 b | 9.9 ± 0.5 | 2.31 ± 0.05 b | |
| 30 | 7.0 ± 1.8 | 13.9 ± 0.6 b | 2.55 ± 0.05 b | 11.8 ± 0.5 b | 2.31 ± 0.06 b | |
| 35 | 7.0 ± 1.8 | 15.0 ± 0.8 b | 2.51 ± 0.05 b | 11.5 ± 0.6 b | 2.17 ± 0.06 b | |
| 40 | 9.5 ± 2.1 b | 19.7 ± 1.5 b | 2.62 ± 0.04 b | 13.3 ± 0.6 b | 2.07 ± 0.06 b | |
2.2.3. Somatic Mutation and Recombination Test (SMART) in Drosophila Melanogaster in Vivo
| Concentration of test solution (%) | Exposure duration 48 h | Exposure duration 120 h | |||||
|---|---|---|---|---|---|---|---|
| No. of wings | Wings with spots (% ± S.E.M.) | Spots/wing (±S.E.M.) | No. of wings | Wings with spots (% ± S.E.M.) | Spots/wing (±S.E.M.) | ||
| Water | 132 | 15.2 ± 3.1 | 0.18 ± 0.03 | 95 | 13.7 ± 3.5 | 0.15 ± 0.03 | |
| Ethanol (5%) | 139 | 17.3 ± 3.2 | 0.19 ± 0.03 | 118 | 13.6 ± 3.1 | 0.14 ± 0.03 | |
| 5,8-DHC | 0.1 | 97 | 26.8 ± 4.5 | 0.27 ± 0.04 | 98 | 22.4 ± 4.2 | 0.25 ± 0.04 a |
| 0.25 | 90 | 21.1 ± 4.3 | 0.22 ± 0.04 | 96 | 27.1 ± 4.5a | 0.30 ± 0.04 a | |
| 0.5 | 96 | 25.0 ± 4.4 | 0.27 ± 0.04 | 97 | 28.9 ± 4.6a | 0.32 ± 0.04 a | |
3. Experimental
3.1. Isolation of 5,8-Dihydroxycoumarin
3.2. Assessment of Antioxidant Potential
3.2.1. DPPH• Radical Scavenging Assay
3.2.2. ABTS•+ Radical Cation Decolorization Assay
3.2.3. Evaluation of Total Phenolic Compounds (TPC)
3.2.4. Ferric Reducing/Antioxidant Power (FRAP) Assay
3.2.5. Oil Oxidation Measurement in Oxipress Apparatus
3.3. Chromosome Aberration and Micronucleus Assay in Wistar Rat Bone Marrow Cells In Vivo
3.3.1. Animals and Treatment Schedule
3.3.2. Chromosome Aberration in Vivo Assay
3.3.3. Micronucleus in Vivo Assay
3.4. Cytogenetic Test in Human Lymphocytes in Vitro
3.5. Somatic Mutation and Recombination Test (SMART) in Drosophila Melanogaster in Vivo
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Hoult, J.R.S.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutical potential. Gen. Pharmacol. 1996, 27, 713722. [Google Scholar]
- Bilgin, H.M.; Atmaca, M.; Obay, B.D.; Ozekinci, S.; Tasdemir, E.; Ketani, A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride-induced acute hepatotoxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 325–330. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Voora, D.; McLeod, H.L.; Eby, C.; Gage, B.F. The pharmacogenetics of coumarin therapy. Pharmacogenomics 2005, 6, 503–513. [Google Scholar] [CrossRef]
- Wu, C.R.; Huang, M.Y.; Lin, Y.T.; Ju, H.Y.; Ching, H. Antioxidant properties of cortex Fraxinis and its simple coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Pukalskas, A.; van Beek, T.A.; Venskutonis, P.R.; Linssen, J.P.H.; van Veldhuizen, A.; de Groot, A. Identification of radical scavengers in sweet grass (Hierochloe odorata). J. Agric. Food Chem. 2002, 50, 2914–2919. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, N.; Ibrahim, M.; Ahmed, E.; Rasool, M.; Malik, A.; Moazzam, M. Further phytochemical studies on Aerva persica. J. Chem. Soc. Pak. 2009, 31, 126–130. [Google Scholar]
- Bandonienė, D.; Pukalskas, A.; Venskutonis, R.; Gruzdienė, D. Preliminary screening of antioxidant activity of some plant extracts in rapeseed oil. Food Res. Int. 2000, 33, 785–791. [Google Scholar] [CrossRef]
- Zainuddin, A.; Pokorny, J.; Venskutonis, R. Antioxidant activity of sweetgrass (Hierochloe odorata Wahlnb.) extract in lard and rapeseed oil emulsions. Nahrung 2002, 46, 15–17. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Rad. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Nemeikaitė-Čėnienė, A.; Marozienė, A.; Pukalskas, A. Redox properties of novel antioxidant 5,8-dihydroxycoumarin: Implications for its prooxidant cytotoxicity. Z. Naturforsch. C. J. Biosci. 2005, 60, 849–854. [Google Scholar]
- EFSA (European Food Safety Authority). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contacts with Food (AFC) on a request from the Commission related to coumarin. EFSA J. 2004, 104, 1–36.
- Api, A.M. Lack of effect of coumarin on the formation of micronuclei in an in vivo mouse micronucleus assay. Food Chem. Toxicol. 2001, 39, 837–841. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). Toxicology and carcinogenesis studies of coumarin (CAS No. 91–64–5) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 1993, 422, 1–340.
- Lin, H.C.; Tsai, S.H.; Chen, C.S.; Chang, Y.C.; Lee, C.M.; Lai, Z.Y.; Lin, C.M. Structure–activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008, 75, 1416–1425. [Google Scholar] [CrossRef]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hasumi, K.; Woo, J.-T.; Nagai, K.; Wachi, M. Generation of hydrogen peroxide primarily contributes to the induction of Fe (II)-dependent apoptosis in Jurkat cells by (D) - epigallocatechin gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their prevention and anticancer properties. Free Rad. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Čėnas, N.; Dičkancaitė, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Rad. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Boersma, M.G.; Vervoort, J.; Szymusiak, H.; Lemanska, K.; Tyrakowska, B.; Čėnas, N.; Segura-Aguilar, J.; Rietjens, I.M.C.M. Regioselectivity and reversibility of the glutathione conjugation of quercetin quinone methide. Chem. Res. Toxicol. 2000, 13, 185–191. [Google Scholar]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 326–329. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Shukla, Y.; Arora, A.; Taneja, P. Antimutagenic potential of cumarin on chromosomal aberrations in Wistar rats. Mutat. Res. 2002, 515, 197–202. [Google Scholar] [CrossRef]
- Kocak, N.; Üstün, H.; Gülkaç, M.D.; Kanli, A.Ö.; Borazan, A.; Yilmaz, A. Effect of 1α,25-dihydroxyvitamin D3 on doxorubicin-induced chromosomal aberrations in rat bone marrow cells. Acta Oncol. 2004, 43, 204–208. [Google Scholar] [CrossRef]
- De Souza, A.B.; Souza, L.M.S.; Carvalo, J.C.T.; Maistro, E.L. No clastogenic activity of Caesalpinia ferrea Mart. (Leguminosae) extract on bone marrow cells of Wistar rats. Genet. Mol. Biol. 2006, 29, 380–383. [Google Scholar] [CrossRef]
- Espósito, A.V.; Pereira, D.M.V.; Rocha, L.M.; Carvalho, J.C.T.; Maistro, E.L. Evaluation of the genotoxic potencial of the Hypericum brasiliense (Guttiferae) extract in mammalian cell system in vivo. Genet. Mol. Biol. 2005, 28, 152–155. [Google Scholar] [CrossRef]
- De Mattos, J.C.P.; de Matos, V.C.; Rodriges, M.P.; de Oliveria, M.B.N.; Dantas, F.J.S.; Santos-Filho, S.D.; Bernardo-Filho, M.; Calderia-de-Araujo, A. Evaluation of deoxyribonucleic acid toxicity induced by the radiopharmaceutical 99mTechnetium-methylenediphosphonic acid and by stannous chloride in Wistar rats. Molecules 2012, 17, 1297–12983. [Google Scholar]
- Hamada, S.; Yamasaki, K.; Nakanishi, S.; Omori, T.; Serikawa, T.; Hayashi, M. Evaluation of the general suitability of the rat for the micronucleus assay: the effect of cyclophosphamide in 14 strains. Mutat. Res. 2001, 495, 127–134. [Google Scholar] [CrossRef]
- Felter, S.P.; Vassallo, J.D.; Carlton, B.D.; Daston, G.P. A safety assessment of coumarin taking into account species-specificity of toxicokinetics. Food Chem. Toxicol. 2006, 44, 462–475. [Google Scholar] [CrossRef]
- Morris, D.L.; Ward, J.B. Coumarin inhibits micronuclei formation induced by benzo(a)pyrene in male but not female ICR mice. Environ. Mol. Mutagen. 1992, 19, 132–138. [Google Scholar] [CrossRef]
- Edwards, A.J.; Price, R.J.; Renwick, A.B.; Lake, B.G. Lack of effect of coumarin on unscheduled DNA synthesis in the in vivo rat hepatocyte DNA Repair Assay. Food Chem. Toxicol. 2000, 38, 403–409. [Google Scholar] [CrossRef]
- Swenberg, J.A. Covalent Binding Index Study on Coumarin. In Report of Laboratory of Molecular Carcinogenesis and Mutagenesis; University of North Carolina: Chapel Hill, NC, US, April 2003; Submitted by European Flavour and Fragrance Association (EFFA): Brussels, Belgium. [Google Scholar]
- Baskaran, N.; Rajasekaran, D.; Manoharan, S. Coumarin protects 7,12-dimethylbenz(a)anthracene-induced genotoxicity in the bone marrow cells of golden Syrian hamsters. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011, 1, 167–173. [Google Scholar] [CrossRef]
- Madari, H.; Panda, D.; Wilson, L.; Jacobs, R.S. Dicoumarol: A unique microtubule stabilizing natural product that is synergistic with taxol. Cancer Res. 2003, 63, 1214–1220. [Google Scholar]
- Podbielkowska, M.; Piwocka, M.; Waszkowska, E.; Waleza, M.; Zobel, A.M. Effect of coumarin and its derivatives on mitosis and ultrastructure of meristematic cells. Int. J. Pharm. 1995, 33, 7–15. [Google Scholar] [CrossRef]
- Lazutka, J.R. Replication index in cultured human lymphocytes: methods for the statistical analysis and possible role in genetic toxicology. Environ. Mol. Mutagen. 1991, 17, 188–195. [Google Scholar] [CrossRef]
- Galloway, S.M.; Armstrong, M.J.; Reuben, C.; Colman, S.; Brown, B.; Cannon, C.; Bloom, A.D.; Nakamura, F.; Ahmed, M.; Duk, S.; et al. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluation of 108 chemicals. Environ. Mol. Mutagen. 1987, 10, 1–175. [Google Scholar]
- Sasaki, Y.F.; Imanishi, H.; Ohta, T.; Shirasu, Y. Effects of antimutagenic flavorings on SCEs induced by chemical mutagenesis in Chinese hamster cells. Mutat. Res. 1987, 188, 313–318. [Google Scholar]
- Sasaki, Y.F.; Imanishi, H.; Watanabe, M.; Ohta, T.; Shirasu, Y. Suppressing effect of antimutagenic flavorings on chromosome aberrations induced by UV-light or X-rays in cultured Chinese hamster cells. Mutat. Res. 1990, 229, 1–10. [Google Scholar] [CrossRef]
- Kaya, B.; Marcos, R.; Yanikoglu, A.; Creus, A. Evaluation of the genotoxicity of four herbicides in the wing spot test of Drosophila melanogaster using two different strains. Mutat. Res. 2004, 557, 53–62. [Google Scholar] [CrossRef]
- Rojas-Molina, M.; Campos-Sánchez, J.; Analla, M.; Munoz-Serrano, A.; Alonso-Moraga, A. Genotoxicity of vegetable cooking oils in the Drosophila wing spot test. Environ. Mol. Mutagen. 2005, 45, 90–95. [Google Scholar] [CrossRef]
- Tellez, M.G.O.; Rodriguez, H.B.; Olivares, G.Q.; Sortibran, A.N.C.; Cetto, A.A.; Rodriguez-Arnaiz, R. A phytotherapeutic extract of Equisetum myriochaetum is not genotoxic either in vivo wing somatic test of Drosophila melanogaster or in the in vitro human micronucleus test. J. Ethnopharmacol. 2007, 111, 182–189. [Google Scholar] [CrossRef]
- Würgler, F.E.; Vogel, E.W. In Vivo mutagenicity testing using somatic cells of Drosophila melanogaster. In Chemical Mutagens, Principles and Methods for their Detection; De Serres, F.J., Ed.; Plenum Press: New York, NY, USA, London, UK, 1986; pp. 1–72. [Google Scholar]
- Graf, U.; Spano, M.A; Rincon, J.G.; Abraham, S.K.; de Andrade, H.H. The wing somatic mutation and recombination test (SMART) in Drosophila melanogaster: An efficient tool for the detection of genotoxic activity of pure compounds or complex mixtures as well as for studies on antigenotoxicity. Afr. Newslett. Occup. Health Safety 1996, 6, 9–13. [Google Scholar]
- Pukalskas, A. Isolation, Identification and activity of natural antioxidants from sweet grass (Hierochloe odorata), Costmary (Chrysanthemum balsamita) and horehound (Marrubium vulgare), cultivated in Lithuania. PhD Thesis, Wageningen University, Wageningen, The Netherlands, 2008. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Folin, C.; Ciocalteu, V. Tyrosine and tryptophane determination in protein. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of, antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Mavournin, K.H.; Blakey, D.H.; Cimino, M.C.; Salamone, M.F.; Heddle, J.A. The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the US Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1990, 236, 29–80. [Google Scholar]
- Krishna, G.; Theiss, J.C. Concurrent analysis of cytogenetic damage in vivo: A multiple endpoin-multiple tissue approach. Environ. Mol. Mutagen. 1995, 25, 314–320. [Google Scholar] [CrossRef]
- Slapsyte, G.; Mierauskiene, J.; Morkunas, V.; Prasmickiene, G.; Didziapetriene, J. Modifying effects of sodium selenite on adriamycin and cyclophosphamide induced chromosome damage and changes of antioxidant status in rats. Trace Elem. Electroly. 2007, 24, 235–243. [Google Scholar]
- Savage, J.R. Classification and relationships of induced chromosomal structural changes. J. Med. Genet. 1976, 13, 103–122. [Google Scholar] [CrossRef]
- Schmid, W. The micronucleus test. Mutat. Res. 1975, 31, 9–15. [Google Scholar] [CrossRef]
- Starke, D.W.; Mieyal, J.J. Haemoglobin catalysis of a monooxygenase-like aliphatic hydroxylation reaction. Biochem. Pharmacol. 1989, 38, 201–204. [Google Scholar] [CrossRef]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Graf, U.; van Schaik, N. Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. 1992, 271, 59–67. [Google Scholar] [CrossRef]
- Van Iersel, M.L.; Verhagen, H.; van Bladeren, P.J. The role of biotransformation in dietary (anti)carcinogenesis. Mutat. Res. 1999, 443, 259–270. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 5,8-dihydroxycoumarin are available from the authors (P.R.V.).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Slapšytė, G.; Dedonytė, V.; Lazutka, J.R.; Mierauskienė, J.; Morkūnas, V.; Kazernavičiūtė, R.; Pukalskas, A.; Venskutonis, P.R. Evaluation of the Biological Activity of Naturally Occurring 5,8-Dihydroxycoumarin. Molecules 2013, 18, 4419-4436. https://doi.org/10.3390/molecules18044419
Slapšytė G, Dedonytė V, Lazutka JR, Mierauskienė J, Morkūnas V, Kazernavičiūtė R, Pukalskas A, Venskutonis PR. Evaluation of the Biological Activity of Naturally Occurring 5,8-Dihydroxycoumarin. Molecules. 2013; 18(4):4419-4436. https://doi.org/10.3390/molecules18044419
Chicago/Turabian StyleSlapšytė, Gražina, Veronika Dedonytė, Juozas R. Lazutka, Jūratė Mierauskienė, Vaidotas Morkūnas, Rita Kazernavičiūtė, Audrius Pukalskas, and Petras Rimantas Venskutonis. 2013. "Evaluation of the Biological Activity of Naturally Occurring 5,8-Dihydroxycoumarin" Molecules 18, no. 4: 4419-4436. https://doi.org/10.3390/molecules18044419