Two New Monoterpene Glycosides from Qing Shan Lu Shui Tea with Inhibitory Effects on Leukocyte-Type 12-Lipoxygenase Activity
Abstract
:1. Introduction
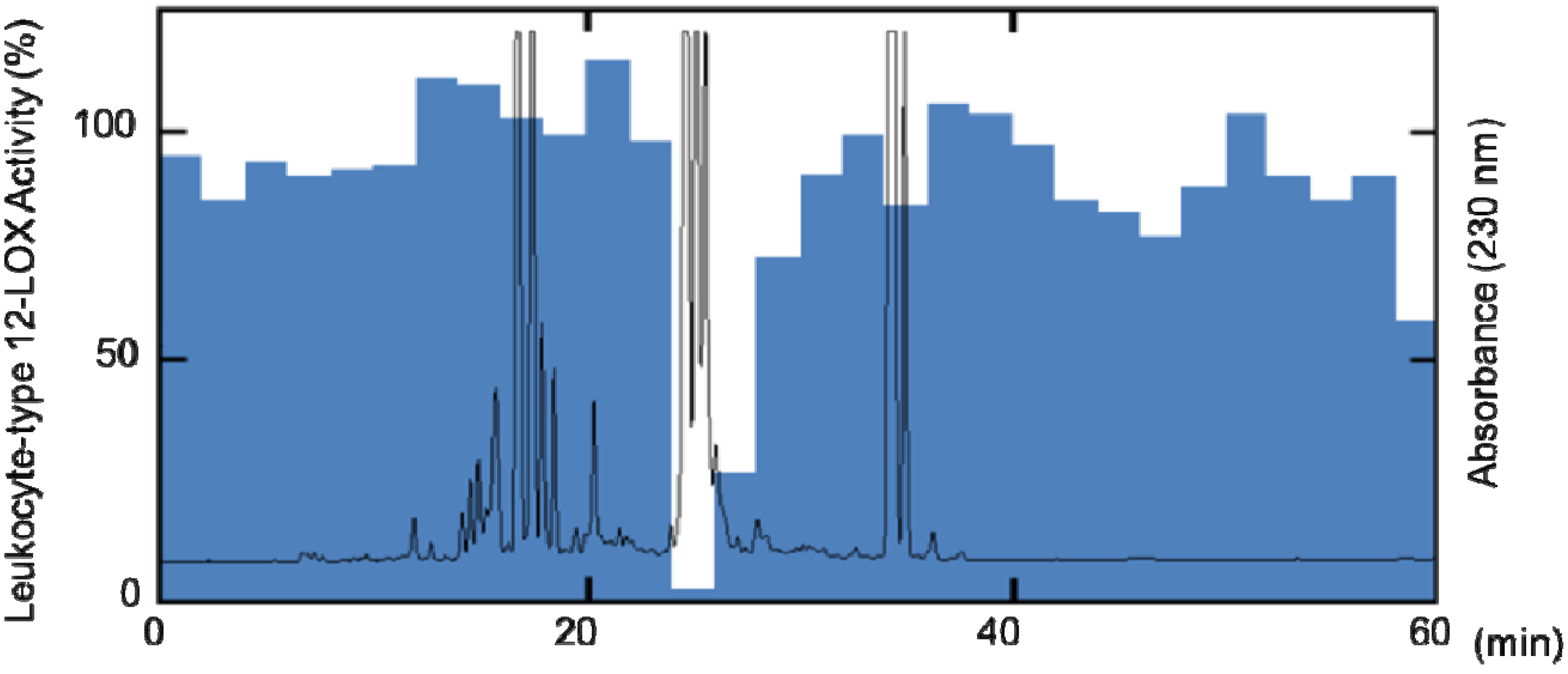
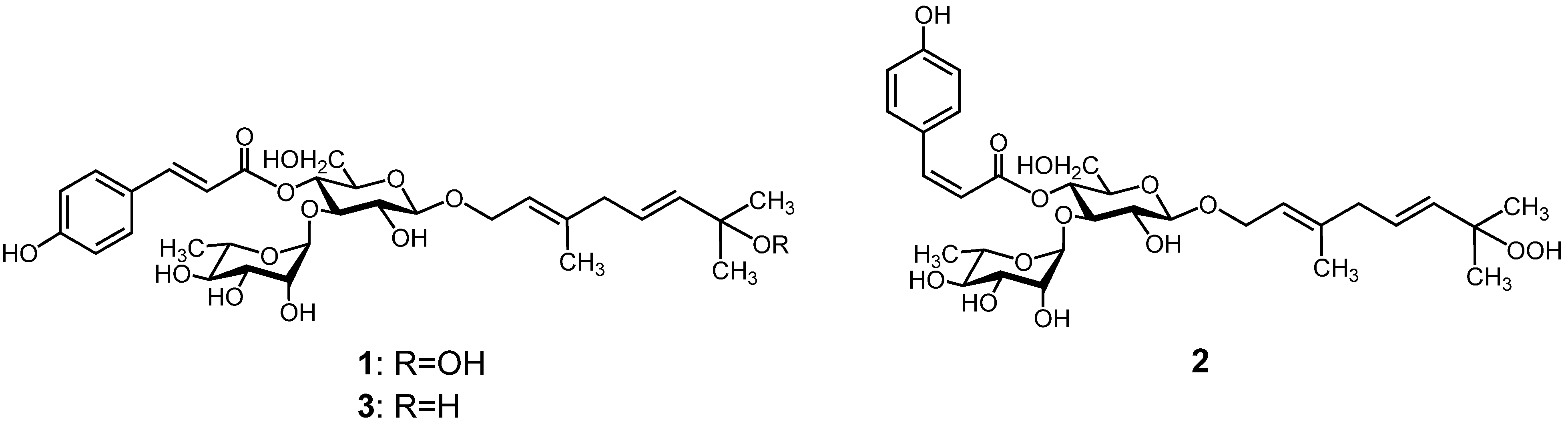
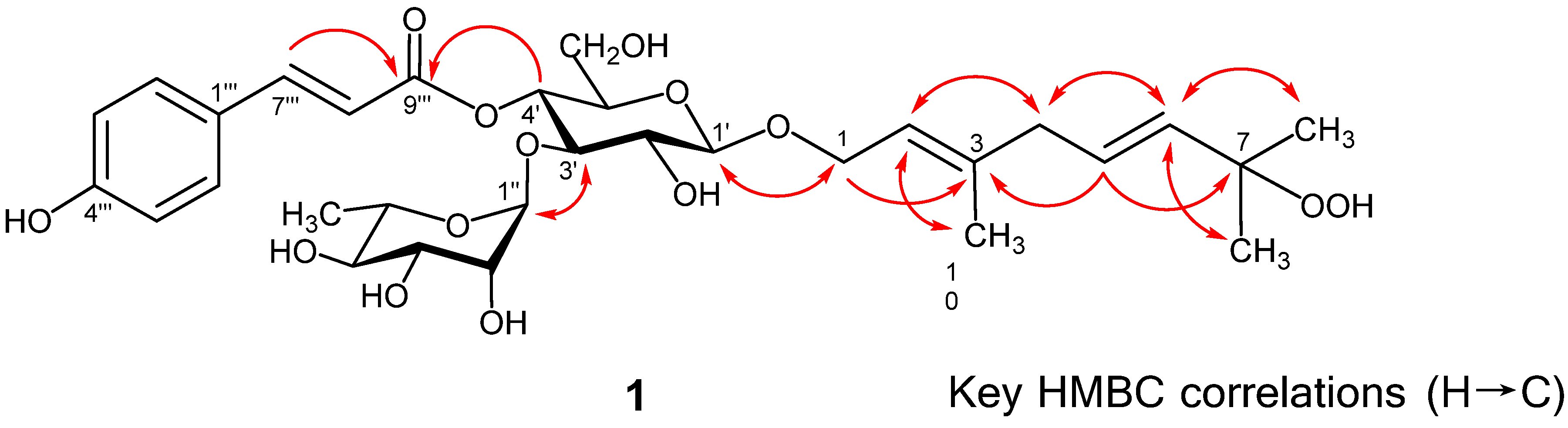
2. Results and Discussion
| Chinese Teas | IC50 (μg/mL) | EGCg | Total Catechins |
|---|---|---|---|
| (mg/g dried leaves) | |||
| Zhuya | 2.4 | 87.4 | 136.5 |
| Man Jian | 6.7 | 38.4 | 63.4 |
| Ganlu | 6.9 | 61.3 | 100.7 |
| Longjin Cha | 9.0 | 63.3 | 100.6 |
| Emei Mao Jian | 13.5 | 58.9 | 95.5 |
| Tie Guan Yin | 16.6 | 18.6 | 39.1 |
| Qing Shan Lu Shui | 19.3 | 0.6 | 0.7 |
| Puer San Cha | 20.0 | 20.8 | 41.3 |
| Tou Cha | 21.2 | 16.3 | 51.4 |
| Tian Cha | 26.8 | <0.1 | 0.4 |
| Hainan Kudig | 38.1 | <0.1 | 0.1 |
| Qin Hua | >100 | <0.1 | 0.1 |
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| Monoterpene | ||||
| 1 | 4.21 dd (7.2, 12) | 65.0 | 4.20 dd (7.2, 12) | 65.9 |
| 4.33 dd (6, 12) | 4.32 dd (6, 12) | |||
| 2 | 5.37 dd (6, 7.2) | 121.4 | 5.36 dd (6, 7.2) | 122.2 |
| 3 | 138.7 | 139.6 | ||
| 4 | 2.75 d (6.6) | 42.2 | 2.74 d (6.6) | 43.1 |
| 5 | 5.61 dt (15.6, 6.6) | 136.7 | 5.61 dt (15.6, 6.6) | 137.6 |
| 6 | 5.67 d (15.6) | 127.0 | 5.66 d (15.6) | 127.8 |
| 7 | 80.6 | 81.4 | ||
| 8 | 1.28 s | 24.1 | 1.27 s | 24.9 |
| 9 | 1.28 s | 24.1 | 1.27 s | 24.9 |
| 10 | 1.67 s | 15.6 | 1.65 s | 16.5 |
| Glucosyl | ||||
| 1′ | 4.40 d (7.2) | 101.4 | 4.38 d (8.4) | 102.3 |
| 2′ | 3.42 dd (7.2, 9) | 75.3 | 3.41 dd (8.4, 9) | 76.1 |
| 3′ | 3.87 t (9) | 78.7 | 3.83 t (9) | 79.7 |
| 4′ | 4.89 t (9) | 69.4 | 4.86 t (9) | 70.1 |
| 5′ | 3.52 m | 75.1 | 3.48 m | 75.8 |
| 6′ | 3.59 m, 3.52 m | 61.5 | 3.59 m, 3.50 m | 62.4 |
| Rhamnosyl | ||||
| 1″ | 5.29 d (1.8) | 100.9 | 5.28 d (1.8) | 101.9 |
| 2″ | 3.86 m | 71.1 | 3.87 m | 71.9 |
| 3″ | 3.52 m | 71.3 | 3.54 dd (3, 9.6) | 72.2 |
| 4″ | 3.30 t (9) | 72.7 | 3.32 t (9.6) | 73.6 |
| 5″ | 3.62 dd (6.6, 9) | 68.5 | 3.65 dd (6.6, 9.6) | 69.4 |
| 6″ | 1.09 d (6.6) | 17.6 | 1.14 d (6.6) | 18.3 |
| Coumaroyl | ||||
| 1′″ | 126.1 | 127.2 | ||
| 2′″ | 7.55 d (8.4) | 130.2 | 7.79 d (8.4) | 134.1 |
| 3′″ | 6.89 d (8.4) | 115.8 | 6.83 d (8.4) | 115.7 |
| 4′″ | 159.9 | 159.8 | ||
| 5′″ | 6.89 d (8.4) | 115.8 | 6.83 d (8.4) | 115.7 |
| 6′″ | 7.55 d (8.4) | 130.2 | 7.79 d (8.4) | 134.1 |
| 7′″ | 7.64 d (15.6) | 145.5 | 6.95 d (13.2) | 145.9 |
| 8′″ | 6.35 d (15.6) | 114.2 | 5.78 d (13.2) | 115.9 |
| 9′″ | 166.2 | 166.0 | ||
3. Experimental
3.1. General
3.2. Quantification of Catechins in Extracts of Chinese Teas
3.3. Leukocyte-type 12-LOX Inhibitory Assay
3.4. Extraction and Isolation
 −81.2° (c 0.3, MeOH); UV λmax (MeOH) nm (log ε): 316 (4.40); 1H- and 13C-NMR, see Table 2; HRESIMS m/z 639.2665 [M−H]−, calcd. for C31H44O14-H, 639.2658.
−81.2° (c 0.3, MeOH); UV λmax (MeOH) nm (log ε): 316 (4.40); 1H- and 13C-NMR, see Table 2; HRESIMS m/z 639.2665 [M−H]−, calcd. for C31H44O14-H, 639.2658. −88.8° (c 0.18, MeOH); UV λmax (MeOH) nm (log ε): 315 (4.81); 1H- and 13C-NMR, see Table 2; HRESIMS m/z 639.2641 [M−H]−, calcd. for C31H44O14-H, 639.2658.
−88.8° (c 0.18, MeOH); UV λmax (MeOH) nm (log ε): 315 (4.81); 1H- and 13C-NMR, see Table 2; HRESIMS m/z 639.2641 [M−H]−, calcd. for C31H44O14-H, 639.2658.4. Conclusions
Acknowledgments
References
- Takahashi, Y.; Zhu, H.; Yoshimoto, T. Essential roles of lipoxygenases in LDL oxidation and development of atherosclerosis. Antioxid. Redox Signal 2005, 7, 425–431. [Google Scholar] [CrossRef]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hosokawa, T.; Morinaka, T.; Irino, S.; Hirano, S.; Kobayashi, H.; Yoshioka, A.; Suzuki-Yamamoto, T.; Yokoro, M.; Kimoto, M.; et al. Antiatherogenic effect of guava leaf extracts inhibiting leucocyte-type 12-lipoxygenase activity. Food Chem. 2012, 131, 1069–1075. [Google Scholar] [CrossRef]
- Trevisanato, S.I.; Kim, Y.I. Tea and health. Nutr. Rev. 2000, 58, 1–10. [Google Scholar] [CrossRef]
- Yamamoto, S.; Katsukawa, M.; Nakano, A.; Hiraki, E.; Nishimura, K.; Jisaka, M.; Yokota, K.; Ueda, N. Arachidonate 12-lipoxygenases with reference to their selective inhibitors. Biochem. Biophys. Res. Commun. 2005, 338, 122–127. [Google Scholar] [CrossRef]
- Tian, J.; Sun, H. New monoterpenoid glycosides from Ligustrum robustum. Chin. J. Appl. Environ. Biol. 1999, 5, 501–506. [Google Scholar]
- Tian, J.; Zhang, H.-J.; Sun, H.-D.; Pan, L.-T.; Yao, P.; Chen, D.-Y. Monoterpenoid glycosides from Ligustrum robustum. Phytochemistry 1998, 48, 1013–1018. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Ke, J.; Lu, D.; Corke, H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse Kudingcha genotypes from Ilex kudingcha, Ilex cornuta; Ligustrum robustum. J. Agric. Food Chem. 2009, 57, 6082–6089. [Google Scholar] [CrossRef]
- Lau, K.M.; He, Z.D.; Dong, H.; Fung, K.P.; But, P.P. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002, 83, 63–71. [Google Scholar] [CrossRef]
- He, Z.D.; Lau, K.M.; But, P.P.; Jiang, R.W.; Dong, H.; Ma, S.C.; Fung, K.P.; Ye, W.C.; Sun, H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod. 2003, 66, 851–854. [Google Scholar] [CrossRef]
- Fukuda, T.; Kitada, Y.; Chen, X.M.; Yang, L.; Miyase, T. Two new monoterpene glycosides from ku-ding-cha. Inhibitors of acyl-CoA: Cholesterol acyltransferase (ACAT). Chem. Pharm. Bull. 1996, 44, 2173–2176. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Murata, H.; Iinuma, M. New furanocoumarins from the fruits of Melicope triphylla. Heterocycles 2011, 83, 1603–1610. [Google Scholar] [CrossRef]
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. New eudesmane-type sesquiterpenoids and other volatile constituents from the roots of Gynura bicolor DC. Flavour Fragr. J. 2011, 26, 55–64. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Ikeshiro, Y.; Kaneyuki, T.; Konishi, T. Nasunin from eggplant consists of cis-trans isomers of delphinidin 3-[4-(p-coumaroyl)-l-rhamnosyl (1→6)glucopyranoside]-5-glucopyranoside. J. Agric. Food Chem. 2005, 53, 9472–9477. [Google Scholar] [CrossRef]
- Masuda, T.; Murota, Y.; Nakatani, N. 7-Hydroxycoumarin derivatives from the juice of Citrus hassaku. Phytochemistry 1992, 31, 1363–1366. [Google Scholar] [CrossRef]
- Sakashita, T.; Takahashi, Y.; Kinoshita, T.; Yoshimoto, T. Essential involvement of 12-lipoxygenase in regiospecific andstereospecific oxidation of low density lipoprotein by macrophages. Eur. J. Biochem. 1999, 265, 825–831. [Google Scholar] [CrossRef]
- Kawakami, Y.; Nakamura, T.; Hosokawa, T.; Suzuki-Yamamoto, T.; Yamashita, H.; Kimoto, M.; Tsuji, H.; Yoshida, H.; Hada, T.; Takahashi, Y. Antiproliferative activity of guava leaf extract via inhibition of prostaglandin endoperoxide H synthase isoforms. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 239–245. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ito, H.; Otsuki, A.; Mori, H.; Li, P.; Kinoshita, M.; Kawakami, Y.; Tsuji, H.; Fang, D.Z.; Takahashi, Y. Two New Monoterpene Glycosides from Qing Shan Lu Shui Tea with Inhibitory Effects on Leukocyte-Type 12-Lipoxygenase Activity. Molecules 2013, 18, 4257-4266. https://doi.org/10.3390/molecules18044257
Ito H, Otsuki A, Mori H, Li P, Kinoshita M, Kawakami Y, Tsuji H, Fang DZ, Takahashi Y. Two New Monoterpene Glycosides from Qing Shan Lu Shui Tea with Inhibitory Effects on Leukocyte-Type 12-Lipoxygenase Activity. Molecules. 2013; 18(4):4257-4266. https://doi.org/10.3390/molecules18044257
Chicago/Turabian StyleIto, Hideyuki, Akemi Otsuki, Hitomi Mori, Peng Li, Mai Kinoshita, Yuki Kawakami, Hideaki Tsuji, Ding Zhi Fang, and Yoshitaka Takahashi. 2013. "Two New Monoterpene Glycosides from Qing Shan Lu Shui Tea with Inhibitory Effects on Leukocyte-Type 12-Lipoxygenase Activity" Molecules 18, no. 4: 4257-4266. https://doi.org/10.3390/molecules18044257




