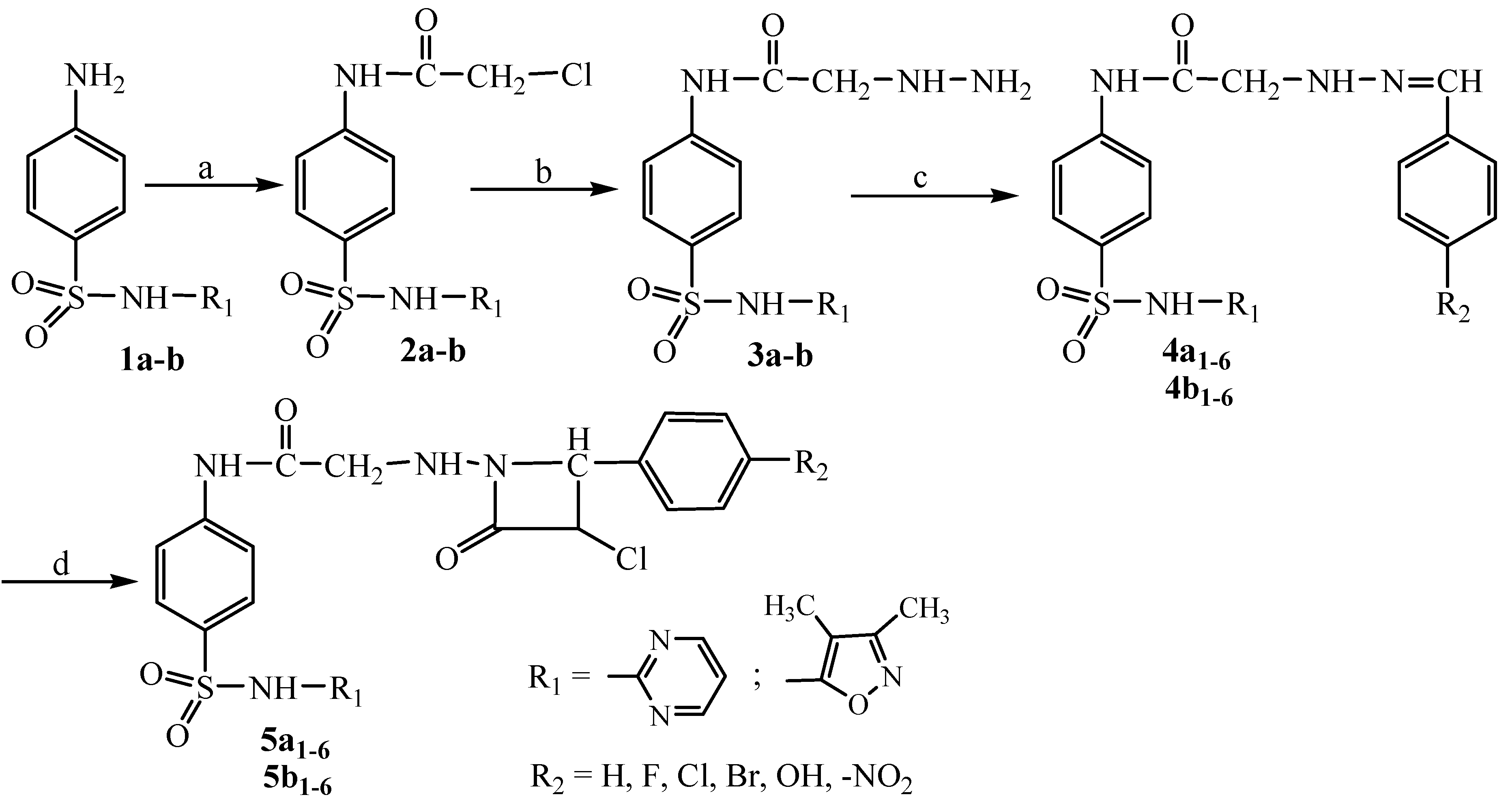
3.2.1. Preparation of N-(arylidene)hydrazinoacetyl Sulfonamides 4a1–6; 4b1–6
To a solution of hydrazinoacetyl sulfonamide derivatives (10 mmol) in ethanol 50% (200 mL), glacial acetic acid (0.5 mL) and the appropriate aldehyde (10 mmol) were added. The mixture was heated under reflux for 8 h, and then it was cooled at room temperature. The solid was filtered off, dried and recrystallized from isopropyl alcohol.
N4-(Benzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a1). Yield 87%, m.p. 200–202 °C; IR (KBr, cm−1): 3450 (-NH), 2853 (CH2-NH), 2924 (=CH- pyrimidine ring), 1693 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1534 (N=CH), 1448 (-C=C- pyrimidine ring), 1313 (C-O), 1128 (-NH-SO2), 1097 (CH aromatic ring), 947 (S-N), 840 (S-C); 1H-NMR δ: 8.72–8.91 (dm, 3H, pyrimidine ring), 8.27 (s, 1H, CO-NH), 8.08 (s, 1H, N=CH), 7.15–7.90 (dm, 9H, Ar-H), 4.19 (s, 1H, -NH-SO2), 3.60 (d, 2H, CH2), 3.43 (m, 1H, HN-N).
N4-(4-Fluorobenzylidene)hydrazinoacetylamino)-N1-(pyrimidin-2-yl)benzensulfonamide (4a2). Yield 90%, m.p. 20–210 °C; IR (KBr, cm−1): 3350 (-NH), 2833 (CH2-NH), 2933 (=CH- pyrimidine ring), 1693 (C=O), 1622 (HN-CO), 1592 (-C=C- aromatic ring), 1535 (N=CH), 1455 (-C=C- pyrimidine ring), 1314 (C-O), 1229 (C-F), 1130 (-NH-SO2), 1099 (CH aromatic ring), 928 (S-N), 836 (S-C); 1H-NMR δ: 8.63-8.90 (dm, 3H, pyrimidine ring), 8.29 (s, 1H, CO-NH), 8.10 (s, 1H, N=CH), 7.1–7.95 (d, 8H, Ar-H), 4.20 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.45 (m, 1H, HN-N).
N4-(4-Chlorobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a3). Yield 83% m.p. 20–207 °C; IR (KBr, cm−1): 3448 (-NH), 2830 (CH2-NH), 2930 (=CH- pyrimidine ring), 1695 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1536 (N=CH), 1496 (-C=C- pyrimidine ring), 1312 (C-O), 1173 (-NH-SO2), 1084 (CH aromatic ring), 940 (S-N), 839 (S-C), 751 (C-Cl); 1H-NMR δ: 8.7–8.89 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 8.15 (s, 1H, N=CH), 7.0–7.76 (d, 8H, Ar-H), 4.17 (s, 1H, -NH-SO2), 3.61 (d, 2H, CH2), 3.40 (m, 1H, HN-N).
N4-(4-Bromobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a4). Yield 81%, m.p. 212–213 °C; IR (KBr, cm−1): 3446 (-NH), 2850 (CH2-NH), 2940 (=CH- pyrimidine ring), 1690 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1535 (N=CH), 1454 (-C=C- pyrimidine ring), 1314 (C-O), 1130 (-NH-SO2), 1079 (CH aromatic ring), 959 (S-N), 840 (S-C), 550 (C-Br); 1H-NMR δ: 8.71–8.89 (dm, 3H, pyrimidine ring), 8.21 (s, 1H, CO-NH), 8.18 (s, 1H, N=CH), 7.19–7.84 (d, 8H, Ar-H), 4.16 (s, 1H, -NH-SO2), 3.56 (d, 2H, CH2), 3.40 (m, 1H, HN-N).
N4-(4-Hydroxybenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a5). Yield 86%, m.p. 208–209 °C; IR (KBr, cm−1): 3505 (OH), 3335 (-NH), 2850 (CH2-NH), 2932 (=CH- pyrimidine ring), 1677 (C=O), 1622 (HN-CO), 1590 (-C=C- aromatic ring), 1539 (N=CH), 1514 (-C=C- pyrimidine ring), 1304 (C-O), 1168 (-NH-SO2), 1078 (CH aromatic ring), 965 (S-N), 839 (S-C); 1H-NMR δ: 8.56–8.89 (dm, 3H, pyrimidine ring), 8.24 (s, 1H, CO-NH), 8.07 (s, 1H, N=CH), 7.35–7.89 (d, 8H, Ar-H), 5.05 (s, 1H, Ar-OH), 4.17 (s, 1H, -NH-SO2), 3.63 (d, 2H, CH2), 3.44 (m, 1H, HN-N).
N4-(4-Nitrobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a6). Yield 75%, m.p. 227–230 °C; IR (KBr, cm−1): 3445 (-NH), 2851 (CH2-NH), 2932 (=CH- pyrimidine ring), 1689 (C=O), 1623 (HN-CO), 1592 (-C=C- aromatic ring), 1536 (N=CH), 1514 (-C=C- pyrimidine ring), 1343 (C-NO2), 1316 (C-O), 1174 (-NH-SO2), 1078 (CH aromatic ring), 951 (S-N), 837 (S-C); 1H-NMR δ: 8.50–8.87 (dm, 3H, pyrimidine ring), 8.21 (s, 1H, CO-NH), 8.06 (s, 1H, N=CH), 7.33–7.90 (d, 8H, Ar-H), 4.16 (s, 1H, -NH-SO2), 3.70 (d, 2H, CH2), 3.41 (m, 1H, HN-N).
N4-(Benzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b1). Yield 87%, m.p. 118–120 °C; IR (KBr, cm−1): 3465 (-NH), 2864 (CH2-NH), 1686 (C=O), 1625 (HN-CO), 1592 (-C=C- aromatic ring), 1518 (N=CH), 1496 (-C=C- oxazole ring), 1310 (C-O), 1151 (-NH-SO2), 1093 (CH aromatic ring), 956 (S-N), 830 (S-C); 1H-NMR δ: 8.41 (s, 1H, CO-NH), 8.12 (s, 1H, N=CH), 7.21–7.93 (dm, 9H, Ar-H), 4.62 (s, 1H, -NH-SO2), 3.59 (d, 2H, CH2), 3.24 (m, 1H, HN-N), 2.01–2.38 (s, 6H, 2CH3).
N4-(4-Fluorobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b2). Yield 68%, m.p. 187–199 °C; IR (KBr, cm−1): 3452 (-NH), 2863 (CH2-NH), 1697 (C=O), 1631 (HN-CO), 1601 (-C=C- aromatic ring), 1507 (N=CH), 1482 (-C=C- oxazole ring), 1320 (C-O), 1225 (C-F), 1153 (-NH-SO2), 1097 (CH aromatic ring), 962 (S-N), 824 (S-C); 1H-NMR δ: 8.36 (s, 1H, CO-NH), 8.21 (s, 1H, N=CH), 7.18–7.91 (d, 8H, Ar-H), 4.65 (s, 1H, -NH-SO2), 3.62 (d, 2H, CH2), 3.38 (m, 1H, HN-N), 2.08–2.34 (s, 6H, 2CH3).
N4-(4-Chlorobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b3). Yield 62%, m.p. 175–178 °C; IR (KBr, cm−1): 3451 (-NH), 2850 (CH2-NH), 1702 (C=O), 1622 (HN-CO), 1586 (-C=C- aromatic ring), 1540 (N=CH), 1483 (-C=C- oxazole ring), 1312 (C-O), 1169 (-NH-SO2), 1086 (CH aromatic ring), 957 (S-N), 830 (S-C), 814 (C-Cl); 1H-NMR δ: 8.31 (s, 1H, CO-NH), 8.16 (s, 1H, N=CH), 7.25–7.98 (d, 8H, Ar-H), 4.74 (s, 1H, -NH-SO2), 3.64 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.08–2.40 (s, 6H, 2CH3).
N4-(4-Bromobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b4). Yield 57%, m.p. 219 °C; IR (KBr, cm−1): 3439 (-NH), 2870 (CH2-NH), 1669 (C=O), 1625 (HN-CO), 1591 (-C=C- aromatic ring), 1522 (N=CH), 1481 (-C=C- oxazole ring), 1315 (C-O), 1156 (-NH-SO2), 1095 (CH aromatic ring), 940 (S-N), 832 (S-C), 555 (C-Br); 1H-NMR δ: 8.32 (s, 1H, CO-NH), 8.18 (s, 1H, N=CH), 7.36-7.94 (d, 8H, Ar-H), 4.61 (s, 1H, -NH-SO2), 3.65 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.10–2.32 (s, 6H, 2CH3).
N4-(4-Hydroxybenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b5). Yield 63%, m.p. 190–192 °C; IR (KBr, cm−1): 3495 (C-OH), 3479 (-NH), 2859 (CH2-NH), 1659 (C=O), 1622 (HN-CO), 1593 (-C=C- aromatic ring), 1530 (N=CH), 1476 (-C=C- oxazole ring), 1323 (C-O), 1150 (-NH-SO2), 1094 (CH aromatic ring), 962 (S-N), 825 (S-C); 1H-NMR δ: 8.36 (s, 1H, CO-NH), 8.22 (s, 1H, N=CH), 7.31–7.84 (d, 8H, Ar-H), 4.92 (s, 1H, Ar-OH), 4.62 (s, 1H, -NH-SO2), 3.58 (d, 2H, CH2), 3.42 (m, 1H, HN-N), 2.08–2.32 (s, 6H, 2CH3).
N4-(4-Nitrobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b6). Yield 71%, m.p. 280 °C; IR (KBr, cm−1): 3433 (-NH), 2842 (CH2-NH), 1683 (C=O), 1629 (HN-CO), 1593 (-C=C- aromatic ring), 1516 (N=CH), 1480 (-C=C- oxazole ring), 1342 (C-NO2), 1329 (C-O), 1161 (-NH-SO2), 1106 (CH aromatic ring), 951 (S-N), 838 (S-C); 1H-NMR δ: 8.28 (s, 1H, CO-NH), 8.21 (s, 1H, N=CH), 7.27–7.88 (d, 8H, Ar-H), 4.64 (s, 1H, -NH-SO2), 3.56 (d, 2H, CH2), 3.21 (m, 1H, HN-N), 2.11–2.34 (s, 6H, 2CH3).
3.2.2. Preparation of N-(4-aryl-3-chloro-2-oxoazetidin-1-yl)aminoacetyl Sulfonamides 5a1–6; 5b1–6
To a solution of N-(arylidene)hydrazinoacetyl sulfonamides 4a1–6; 4b1–6 (2 mmol) in anhydrous 1,4-dioxane (50 mL), chloracetyl chloride (3 mmol) and triethylamine (2 mmol) were added dropwise at 0–5 °C. The mixture of reaction was stirred at room temperature for 3 h and the solid (triethylamine hydrochloride) was removed. The solution was heated under reflux for 5 h and then the solvent was evaporated under reduced pressure. The solid product was washed with water (20 mL), filtered off, dried and recrystallized from absolute ethanol. The progress of the reaction was monitored by silica gel coated TLC plates.
N4-(2-Phenyl-3-chloro-4-oxoazetidin-1-yl)aminoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (5a1). Yield 75%, m.p. 218–220 °C; IR (KBr, cm−1): 3450 (-NH), 2869 (CH2-NH), 2945 (=CH- pyrimidine ring), 1745 (CO β-lactam), 1622 (HN-CO), 1591 (-C=C- aromatic ring), 1449 (-C=C- pyrimidine ring), 1314 (C-O), 1131 (-NH-SO2), 1078 (CH aromatic ring), 945 (S-N), 841 (S-C), 635 (C-Cl); 1H-NMR δ: 8.49–8.72 (dm, 3H, pyrimidine ring), 8.25 (s, 1H, CO-NH), 7.21–7.96 (dm, 9H, Ar-H), 5.45 (d, 1H, CH-Ar, azetidinone ring), 5.18 (d, 1H, CH-Cl), 4.27 (s, 1H, -NH-SO2), 3.75 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 170.4 (CONH), 162.8 (CO, β-lactam), 126.7–140.9 (12 aromatic carbons), 118.9 (1C pyrimidine ring), 154.7 (3C pyrimidine ring), 67.8 (CH), 62.7 (CH-Cl), 57.7 (CH2); Anal. calcd for C21H19O4N6S: C 55.87, H 4.24, N 18.61; found: C 56.02, H 4.38, N 18.48.
N4-[2-(4-Fluoro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a2). Yield 90%, m.p. 213–215 °C; IR (KBr, cm−1): 3355 (-NH), 2831 (CH2-NH), 2943 (=CH- pyrimidine ring), 1744 (CO β-lactam), 1624 (HN-CO), 1593 (-C=C- aromatic ring), 1508 (-C=C- pyrimidine ring), 1315 (C-O), 1230 (C-F), 1131 (-NH-SO2), 1100 (CH aromatic ring), 926 (S-N), 836 (S-C), 613 (C-Cl); 1H-NMR δ: 8.47–8.70 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 7.23–7.97 (d, 8H, Ar-H), 5.35 (d, 1H, CH-Ar, azetidinone ring), 5.04 (d, 1H, CH-Cl), 4.37 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 169.2 (CONH), 162.5 (CO, β-lactam), 124.9–141.06 (12 aromatic carbons), 118.8 (1C pyrimidine ring), 151.8 (3C pyrimidine ring), 69.2 (CH), 61.04 (CH-Cl), 55.6 (CH2); Anal. calcd for C21H18O4N6SF: C 53.73, H 3.86, N 17.90; found: C 53.96, H 4.06, N 18.14.
N4-[2-(4-Chloro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a3). Yield 85%, m.p. 201–203 °C; IR (KBr, cm−1): 3355 (-NH), 2830 (CH2-NH), 2944 (=CH- pyrimidine ring), 1745 (CO β-lactam), 1624 (HN-CO), 1592 (-C=C- aromatic ring), 1490 (-C=C- pyrimidine ring), 1314 (C-O), 1174 (-NH-SO2), 1088 (CH aromatic ring), 958 (S-N), 825 (S-C), 610 (C-Cl); 1H-NMR) δ: 8.48–8.74 (dm, 3H, pyrimidine ring), 8.24 (s, 1H, CO-NH), 7.19–7.96 (d, 8H, Ar-H), 5.38 (d, 1H, CH-Ar, azetidinone ring), 5.00 (d, 1H, CH-Cl), 4.4 (s, 1H, -NH-SO2), 3.75 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 167.6 (CONH), 160.5 (CO, β-lactam), 126.7–142.6 (12 aromatic carbons), 120.5 (1C pyrimidine ring), 155.1 (3C pyrimidine ring), 68.7 (CH), 62.8 (CH-Cl), 54.4 (CH2); Anal. calcd for C21H18O4N6SCl: C 51.91, H 3.73, N 17.29; found: C 52.14, H 3.95, N 17.04.
N4-[2-(4-Bromo)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a4). Yield 88%, m.p. 206–208 °C; IR (KBr, cm−1): 3445 (-NH), 2848 (CH2-NH), 2943 (=CH- pyrimidine ring), 1739 (CO β-lactam), 1624 (HN-CO), 1590 (-C=C- aromatic ring), 1485 (-C=C- pyrimidine ring), 1315 (C-O), 1130 (-NH-SO2), 1067 (CH aromatic ring), 961 (S-N), 840 (S-C), 630 (C-Cl), 572 (C-Br); 1H-NMR δ: 8.47–8.71 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 7.16–7.85 (d, 8H, Ar-H), 5.36 (d, 1H, CH-Ar, azetidinone ring), 5.05 (d, 1H, CH-Cl), 4.27 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 165.3 (CONH), 160.7 (CO, β-lactam), 126.6–136.6 (12 aromatic carbons), 118.7 (1C pyrimidine ring), 156.6 (3C pyrimidine ring), 68.2 (CH), 62.09 (CH-Cl), 57.5 (CH2); Anal. calcd for C21H18O4N6SBr: C 47.56, H 3.42, N 15.85; found: C 47.28, H 3.71, N 15.67.
N4-[2-(4-Hydroxy)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a5). Yield 80%, m.p. 231–233 °C; IR (KBr, cm−1): 3497 (C-OH), 3355 (-NH), 2843 (CH2-NH), 2943 (=CH- pyrimidine ring), 1740 (CO β-lactam), 1622 (HN-CO), 1590 (-C=C- aromatic ring), 1495 (-C=C- pyrimidine ring), 1315 (C-O), 1175 (-NH-SO2), 1067 (CH aromatic ring), 961 (S-N), 840 (S-C), 636 (C-Cl), 1H-NMR δ: 8.54–8.75 (dm, 3H, pyrimidine ring), 8.26 (s, 1H, CO-NH), 7.30–7.90 (d, 8H, Ar-H), 5.34 (d, 1H, CH-Ar, azetidinone ring), 5.06 (d, 1H, CH-Cl), 4.95 (s, 1H, Ar-OH), 4.25 (s, 1H, -NH-SO2), 3.64 (d, 2H, CH2), 3.40 (m, 1H, HN-N); 13C-NMR δ: 167.7 (CONH), 160.3 (CO, β-lactam), 114.7–132.1 (12 aromatic carbons), 119.5 (1C pyrimidine ring), 153.4 (3C pyrimidine ring), 72.9 (CH), 63.3 (CH-Cl), 54.2 (CH2); Anal. calcd for C21H19O5N6S: C 53.95, H 4.10, N 17.98; found: C 54.21, H 4.32, N 18.19.
N4-[2-(4-Nitro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a6). Yield 86%, m.p. 200–203 °C; IR (KBr, cm−1): 3350 (-NH), 2846 (CH2-NH), 2942 (=CH- pyrimidine ring), 1743 (CO β-lactam), 1624 (HN-CO), 1593 (-C=C- aromatic ring), 1496 (-C=C- pyrimidine ring), 1343 (C-NO2), 1316 (C-O), 1131 (-NH-SO2), 1079 (CH aromatic ring), 951 (S-N), 839 (S-C), 635 (C-Cl); 1H-NMR) δ: 8.36–8.87 (dm, 3H, pyrimidine ring), 8.29 (s, 1H, CO-NH), 7.19–7.99 (d, 8H, Ar-H), 5.33 (d, 1H, CH-Ar, azetidinone ring), 5.02 (d, 1H, CH-Cl), 4.24 (s, 1H, -NH-SO2), 3.62 (d, 2H, CH2), 3.37 (m, 1H, HN-N); 13C-NMR δ: 168.3 (CONH), 161.9 (CO, β-lactam), 118.5–137.2 (12 aromatic carbons), 118.6 (1C pyrimidine ring), 155.8 (3C pyrimidine ring), 76.1 (CH), 64.3 (CH-Cl), 58.0 (CH2); Anal. calcd for C21H18O6N7S: C 50.80, H 3.65, N 19.75; found: C 50.64, H 3.89, N 19.97.
N4-(2-Phenyl-3-chloro-4-oxoazetidin-1-yl)aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzen-sulfonamide (5b1). Yield 51%, m.p. 212–213 °C; IR (KBr, cm−1): 3342 (-NH), 2860 (CH2-NH), 1743 (CO β-lactam), 1624 (HN-CO), 1591 (-C=C- aromatic ring), 1495 (-C=C- oxazole ring), 1318 (C-O), 1153 (-NH-SO2), 1092 (CH aromatic ring), 957 (S-N), 836 (S-C), 663 (C-Cl); 1H-NMR δ: 8.34 (s, 1H, CO-NH), 7.13–7.86 (dm, 9H, Ar-H), 5.37 (d, 1H, CH-Ar, azetidinone ring), 5.19 (d, 1H, CH-Cl), 4.57 (s, 1H, -NH-SO2), 3.51 (d, 2H, CH2), 3.11 (m, 1H, HN-N), 1.95–2.30 (s, 6H, 2CH3); 13C-NMR δ: 168.5 (CONH), 164.0 (CO, β-lactam), 151.4–155.8 (2C oxazole ring), 101.05 (1C oxazole ring), 118.8–135.3 (12 aromatic carbons), 77.2 (CH), 66.3 (CH-Cl), 59.2 (CH2), 8.48 (2CH3); Anal. calcd for C22H22O5N5S: C 56.40, H 4.73, N 14.95; found: C 56.63, H 4.94, N 15.18.
N4-[2-(4-Fluorophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b2). Yield 76%, m.p. 209–211 °C; IR (KBr, cm−1): 3450 (-NH), 2861 (CH2-NH), 1739 (CO β-lactam), 1633 (HN-CO), 1591 (-C=C- aromatic), 1497 (-C=C- oxazole ring), 1322 (C-O), 1232 (C-F), 1153 (-NH-SO2), 1094 (CH aromatic ring), 961 (S-N), 836 (S-C), 662 (C-Cl); 1H-NMR δ: 8.24 (s, 1H, CO-NH), 7.10–7.85 (d, 8H, Ar-H), 5.38 (d, 1H, CH-Ar, azetidinone ring), 5.23 (d, 1H, CH-Cl), 4.57 (s, 1H, -NH-SO2), 3.57 (d, 2H, CH2), 3.41 (m, 1H, HN-N), 2.02–2.27 (s, 6H, 2CH3); 13C-NMR δ: 169.6 (CONH), 160.3 (CO, β-lactam), 118.6–139.9 (12 aromatic carbons), 99.56 (1C oxazole ring), 151.8–153.3 (2C oxazole ring), 75.9 (CH), 64.2 (CH-Cl), 56.2 (CH2), 10.84 (2CH3); Anal. calcd for C22H21O5N5SF: C 54.31, H 4.35, N 14.40; found: C 54.09, H 4.58, N 14.68.
N4-[2-(4-Chlorophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b3). Yield 72%, m.p. 211–213 °C; IR (KBr, cm−1): 3361 (-NH), 2869 (CH2-NH), 1750 (CO β-lactam), 1620 (HN-CO), 1591 (-C=C- aromatic), 1495 (-C=C oxazole ring), 1318 (C-O), 1153 (-NH-SO2), 1092 (CH aromatic ring), 957 (S-N), 836 (S-C), 663 (C-Cl); 1H-NMR δ: 8.23 (s, 1H, CO-NH), 7.22–7.92 (d, 8H, Ar-H), 5.35 (d, 1H, CH-Ar, azetidinone ring), 5.20 (d, 1H, CH-Cl), 4.68 (s, 1H, -NH-SO2), 3.52 (d, 2H, CH2), 3.33 (m, 1H, HN-N), 2.05–2.36 (s, 6H, 2CH3); 13C-NMR (DMSO) δ in ppm: 170.3 (CONH), 163.6 (CO, β-lactam), 118.8–132.7 (12 aromatic carbons), 100.97 (1C oxazole ring), 150.4–152.7 (2C oxazole ring), 77.4 (CH), 66.3 (CH-Cl), 53.1 (CH2), 8.50 (2CH3); Anal. calcd for C22H21O5N5SCl: C 52.54, H 4.21, N 13.92; found: C 52.38, H 4.08, N 13.71.
N4-[2-(4-Bromophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b4). Yield 80%, m.p. 212–214 °C; IR (KBr, cm−1): 3386 (-NH), 2866 (CH2-NH), 1748 (CO β-lactam), 1626 (HN-CO), 1589 (-C=C- aromatic ring), 1483 (-C=C- oxazole ring), 1315 (C-O), 1158 (-NH-SO2), 1095 (CH aromatic ring), 960 (S-N), 859 (S-C), 660 (C-Cl), 556 (C-Br); 1H-NMR δ: 8.21 (s, 1H, CO-NH), 7.34–7.98 (d, 8H, Ar-H), 5.33 (d, 1H, CH-Ar, azetidinone ring), 5.19 (d, 1H, CH-Cl), 4.56 (s, 1H, -NH-SO2), 3.60 (d, 2H, CH2), 3.36 (m, 1H, HN-N), 2.08–2.28 (s, 6H, 2CH3); 13C-NMR δ: 171.3 (CONH), 160.7 (CO, β-lactam), 128.8–142.7 (12 aromatic carbons), 102.37 (1C oxazole ring), 151.7–153.5 (2C oxazole ring), 78.8 (CH), 67.0 (CH-Cl), 55.0 (CH2), 8.50 (2CH3); Anal. calcd for C22H21O5N5SBr: C 48.27, H 3.87, N 12.79; found: C 48.54, H 3.94, N 12.97.
N4-[2-(4-Hydroxyphenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b5). Yield 55%, m.p. 210–212 °C; IR (KBr, cm−1): 3561 (C-OH), 3461 (-NH), 2855 (CH2-NH), 1746 (CO β-lactam), 1621 (HN-CO), 1590 (-C=C- aromatic), 1480 (-C=C- oxazole ring), 1308 (C-O), 1151 (-NH-SO2), 1093 (CH aromatic ring), 989 (S-N), 835 (S-C), 659 (C-Cl); 1H-NMR δ in ppm: 8.31 (s, 1H, CO-NH), 7.28–7.81 (d, 8H, Ar-H), 5.32 (d, 1H, CH-Ar, azetidinone ring), 5.10 (d, 1H CH-Cl), 4.84 (s, 1H, Ar-OH), 4.57 (s, 1H, -NH-SO2), 3.55 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.04–2.28 (s, 6H, 2CH3); 13C-NMR δ: 175.2 (CONH), 168.2 (CO, β-lactam), 115.8–126.8 (12 aromatic carbons), 101.79 (1C oxazole ring), 148.9–150.5 (2C oxazole ring), 77.3 (CH), 64.8 (CH-Cl), 55.2 (CH2), 8.51 (2CH3); Anal. calcd for C22H22O6N5S: C 54.54, H 4.58, N 14.45; found: C 54.73, H 4.63, N 14.71.
N4-[2-(4-Nitrophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b6). Yield 59%, m.p. 218–220 °C; IR (KBr, cm−1): 3415 (-NH), 2863 (CH2-NH), 1752 (CO β-lactam), 1624 (HN-CO), 1591 (-C=C- aromatic ring), 1490 (-C=C- oxazole ring), 1337 (C-O), 1329 (C-NO2) 1154 (-NH-SO2), 1092 (CH aromatic ring), 987 (S-N), 836 (S-C), 662 (C-Cl); 1H-NMR δ: 8.18 (s, 1H, CO-NH), 7.19–7.86 (d, 8H, Ar-H), 5.36 (d, 1H, CH-Ar, azetidinone ring), 5.20 (d, 1H, CH-Cl), 4.55 (s, 1H, -NH-SO2), 3.49 (d, 2H, CH2), 3.11 (m, 1H, HN-N); 2.09–2.27 (s, 6H, 2CH3); 13C-NMR δ: 170.7 (CONH), 165.1 (CO, β-lactam), 118.7–130.6 (12 aromatic carbons), 101.20 (1C oxazole ring), 148.8–150.4 (2C oxazole ring), 79.1 (CH), 65.4 (CH-Cl), 60.7 (CH2), 9.54 (2CH3); Anal. calcd for C22H21O7N6S: C 51.46, H 4.12, N 16.37; found: C 51.29, H 4.36, N 16.09.