Cholinesterase Enzymes Inhibitors from the Leaves of Rauvolfia Reflexa and Their Molecular Docking Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bioactivity-Guided Isolation
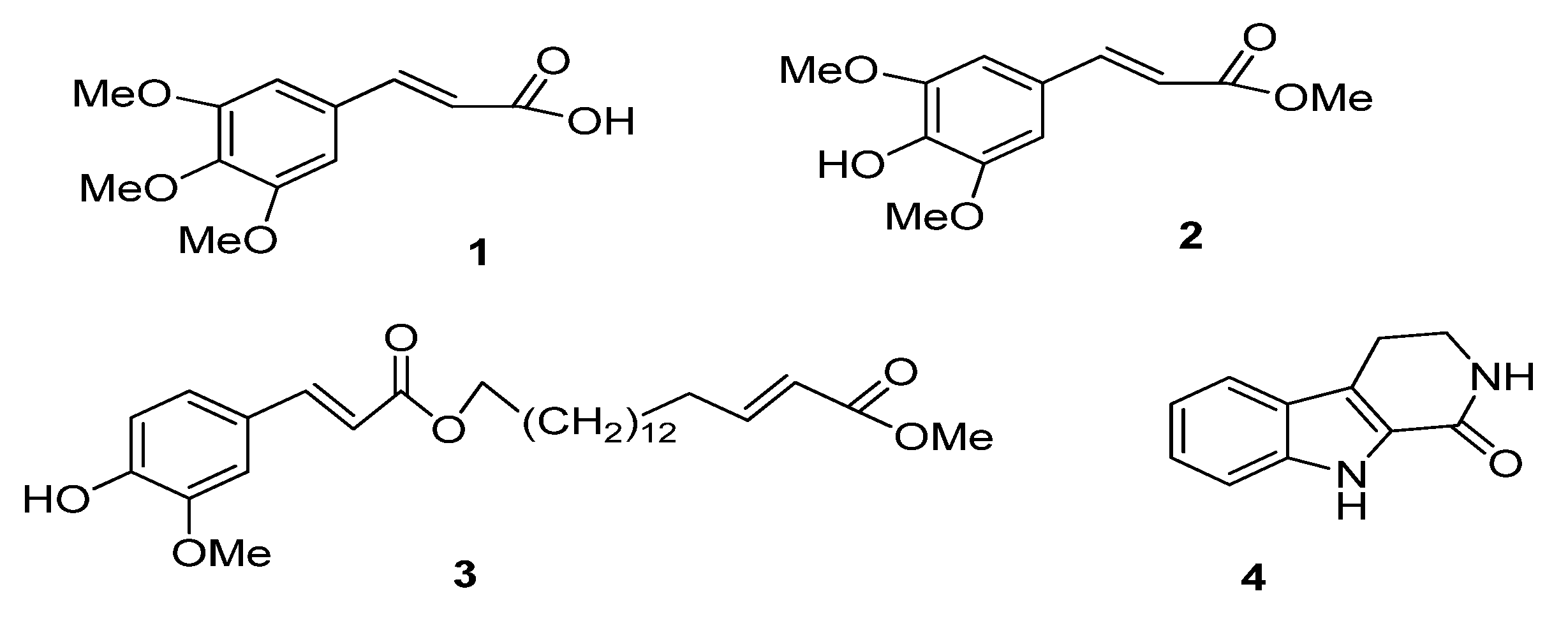

2.2. Cholinesterase Enzymes Inhibitory Activity
| Extract | AChE inhibition, IC50 (μg/mL) | BChE inhibition, IC50 (μg/mL) | Selectivity for | |
|---|---|---|---|---|
| AChE a | BChE b | |||
| Dichloromethane | 14.65 ± 0.32 | 8.49 ± 0.92 | 0.58 | 1.73 |
| Ethanol | 38.40 ± 0.15 | 26.47 ± 2.05 | 0.79 | 1.26 |
| Methanol | 52.23 ± 4.02 | ND | - | - |
| Compound | AChE inhibition, IC50 | BChE inhibition, IC50 | Selectivity for | |||
|---|---|---|---|---|---|---|
| μg/mL | μM | μg/mL | μM | AChE a | BChE b | |
| 1 | 14.32 ± 0.82 | 60.17 ± 14.45 | ND | - | - | - |
| 2 | 37.63 ± 1.42 | 158.06 ± 5.9 | 14.69 ± 1.22 | 61.72 ± 5.14 | 0.39 | 2.56 |
| 3 | 48.99 ± 2.86 | 97.37 ± 5.64 | ND | - | - | - |
| 4 | 15.52 ± 0.68 | 83.38 ± 3.67 | ND | - | - | - |
| Physostigmine | 0.046 | 0.17 | 0.162 | 0.59 | 3.47 | 0.29 |
2.3. Molecular Docking of Bioactive Compounds 1 and 2
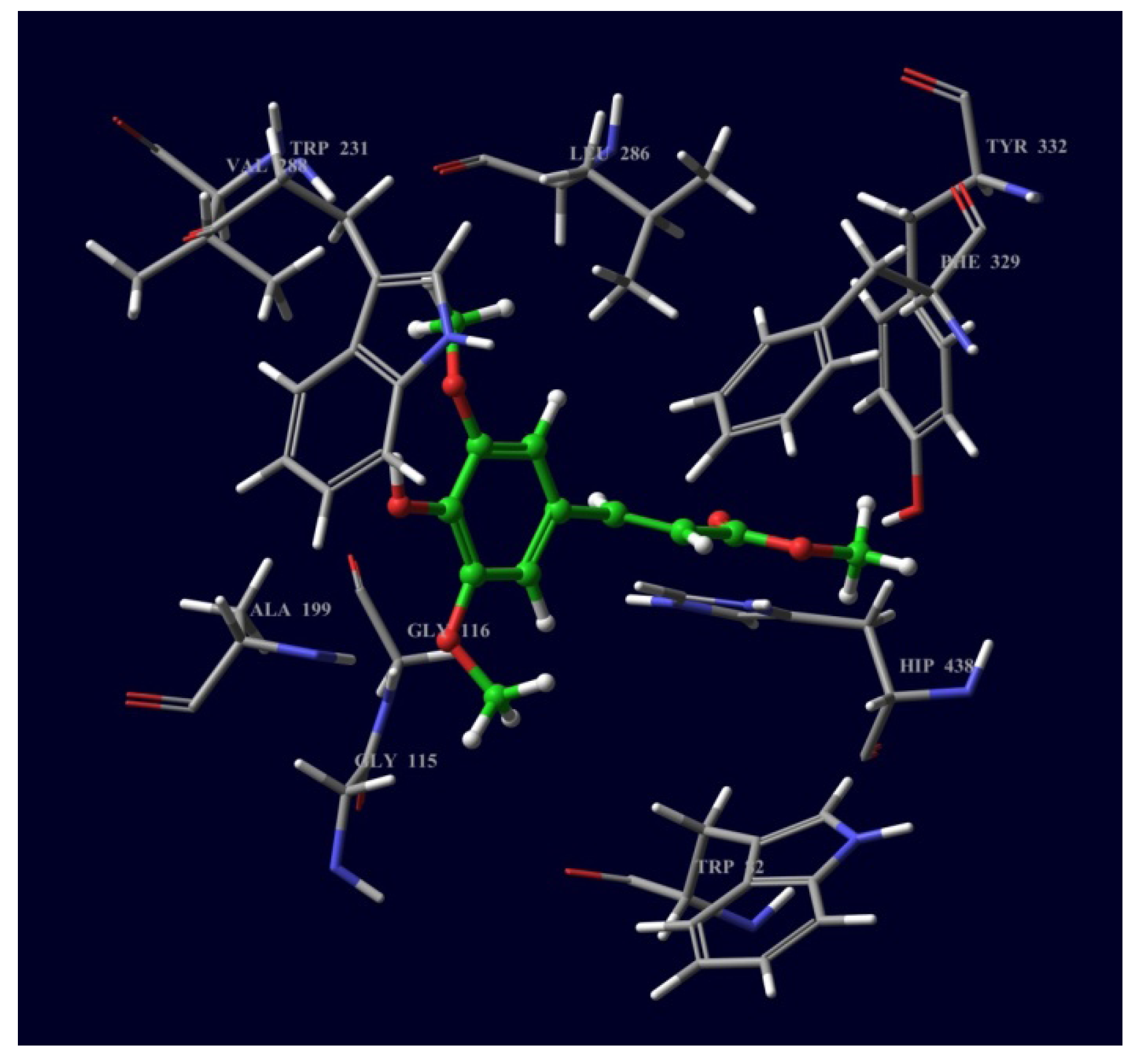
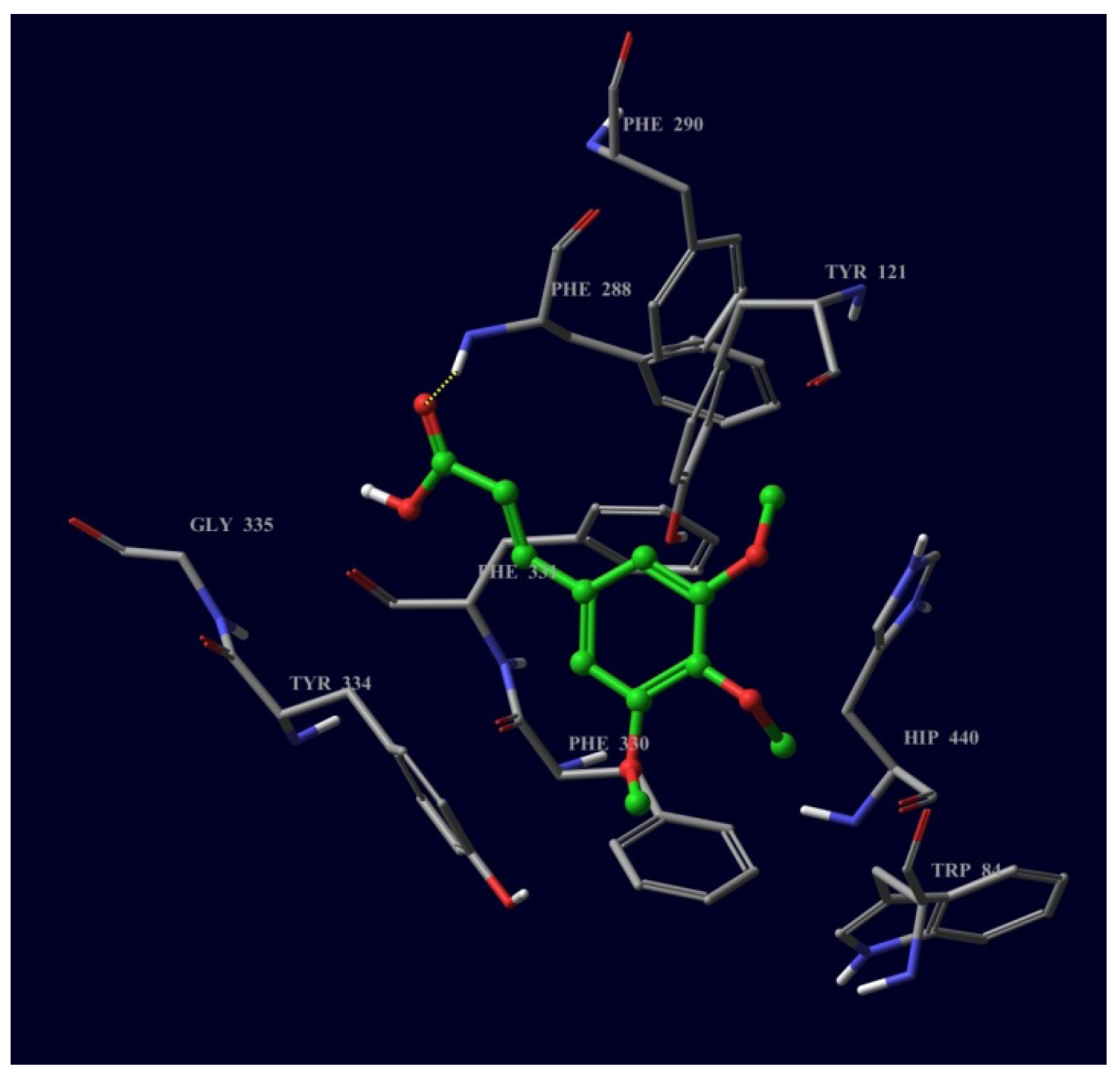
3. Experimental
3.1. Plant Materials
3.2. Enzymes and Chemicals
3.3. General Experimental Procedures
3.4. Preparation of Extract
3.5. Isolation and Characterization of Bioactive Compounds
3.6. Characterization Data
3.7. Cholinesterase Enzymes Inhibitory Assay
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Racchi, M.; Mazzucchelli, M.E.; Porello, G.L.; Govoni, S. Acetylcholinesterase inhibitors: Novel activities of old molecules. Pharmacol. Res. 2004, 50, 103–110. [Google Scholar]
- Massoud, G.; Gauthier, S. Update on the pharamcological treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2010, 8, 69–80. [Google Scholar] [CrossRef]
- Pohanka, M. Cholinesterases, A target of pharmacology and toxicology. Biomed. Papers 2011, 155, 219–223. [Google Scholar] [CrossRef]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat. 2012, 22, 871–886. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin. Inv. Drug 2006, 15, 1–12. [Google Scholar] [CrossRef]
- Schultes, R.E. Plants in treating senile dementia in the Northwest Amazon. J. Ethnopharmacol. 1993, 38, 121–128. [Google Scholar] [CrossRef]
- Mainen, J.M.; Donald, F.O.; Anke, W. Ethnomedicine of the Kagera region, north western Tanzania. Part 3: Plants used in traditional medicine in Kikuku village, Muleba district. J. Ethnobio. Ethnomed. 2012, 8, 14–15. [Google Scholar] [CrossRef]
- Fernando, C.; Luis, O.R.; Vanderlan, D.S.B.; Hosana, M.D.; Gabriela, D.P. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res. 2009, 18, 703–711. [Google Scholar] [CrossRef]
- Da silva, V.C.; De Carvalho, M.G. Chemical constituents from leaves of Palicourea coriacea. J. Nat. Med. 2008, 62, 356–357. [Google Scholar] [CrossRef]
- Fadaei, M.; Hamid, A.H.A. Antioxidant and antimicrobial activities of ferulic acid esters from Ochrosia oppositifoli. Malays. J. Sci. 2011, 30, 154–160. [Google Scholar]
- Zhang, L.S.F.; Yan, L.; Kong, X.R. β-carboline alkaloids from the leaves of Trigonostemon lii Y.T. Chang. Bioorg. Med. Chem. Lett. 2012, 22, 2296–2299. [Google Scholar] [CrossRef]
- Wong, K.K.; Ngo, J.C.; Liu, S.; Lin, H.Q.; Hu, C.; Shaw, P.C.; Wan, D.C. Interaction study of two diterpenes, Cryptotanshinone and dihydrotanshinone, to human acetylcholinesterase and butyrylcholinesterase by molecular docking and kinetic analysis. Chem. Biol. Interact. 2010, 187, 335–339. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. 2009, 91, 554–559. [Google Scholar] [CrossRef]
- Obregon, A.D.C.; Schetinger, M.R.C.; Correa, M.M.; Morsch, V.M.; Da Silva, J.E.P.; Martins, M.A.P.; Bonacorso, H.G.; Zanatta, N. Effects per se of organic solvents in the cerebral acetylcholinesterase of rats. Neurochem. Res. 2005, 30, 379–384. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fadaeinasab, M.; Hadi, A.H.A.; Kia, Y.; Basiri, A.; Murugaiyah, V. Cholinesterase Enzymes Inhibitors from the Leaves of Rauvolfia Reflexa and Their Molecular Docking Study. Molecules 2013, 18, 3779-3788. https://doi.org/10.3390/molecules18043779
Fadaeinasab M, Hadi AHA, Kia Y, Basiri A, Murugaiyah V. Cholinesterase Enzymes Inhibitors from the Leaves of Rauvolfia Reflexa and Their Molecular Docking Study. Molecules. 2013; 18(4):3779-3788. https://doi.org/10.3390/molecules18043779
Chicago/Turabian StyleFadaeinasab, Mehran, A. Hamid A. Hadi, Yalda Kia, Alireza Basiri, and Vikneswaran Murugaiyah. 2013. "Cholinesterase Enzymes Inhibitors from the Leaves of Rauvolfia Reflexa and Their Molecular Docking Study" Molecules 18, no. 4: 3779-3788. https://doi.org/10.3390/molecules18043779
APA StyleFadaeinasab, M., Hadi, A. H. A., Kia, Y., Basiri, A., & Murugaiyah, V. (2013). Cholinesterase Enzymes Inhibitors from the Leaves of Rauvolfia Reflexa and Their Molecular Docking Study. Molecules, 18(4), 3779-3788. https://doi.org/10.3390/molecules18043779




