Article Synthesis and Trypanocidal Activity of Novel 2,4,5-Triaryl-N-Hydroxylimidazole Derivatives
Abstract
:1. Introduction
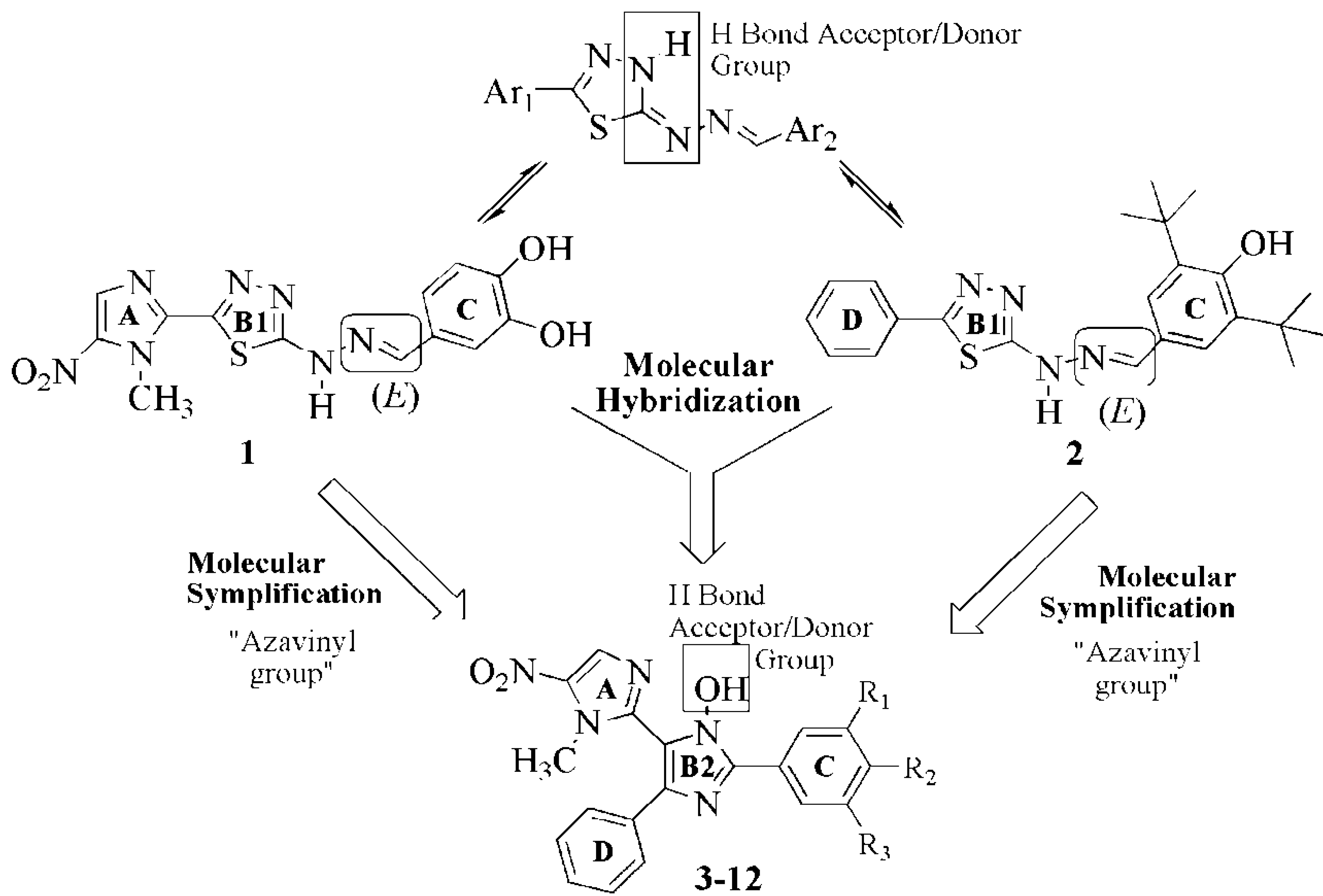
2. Results and Discussion
2.1. Chemistry
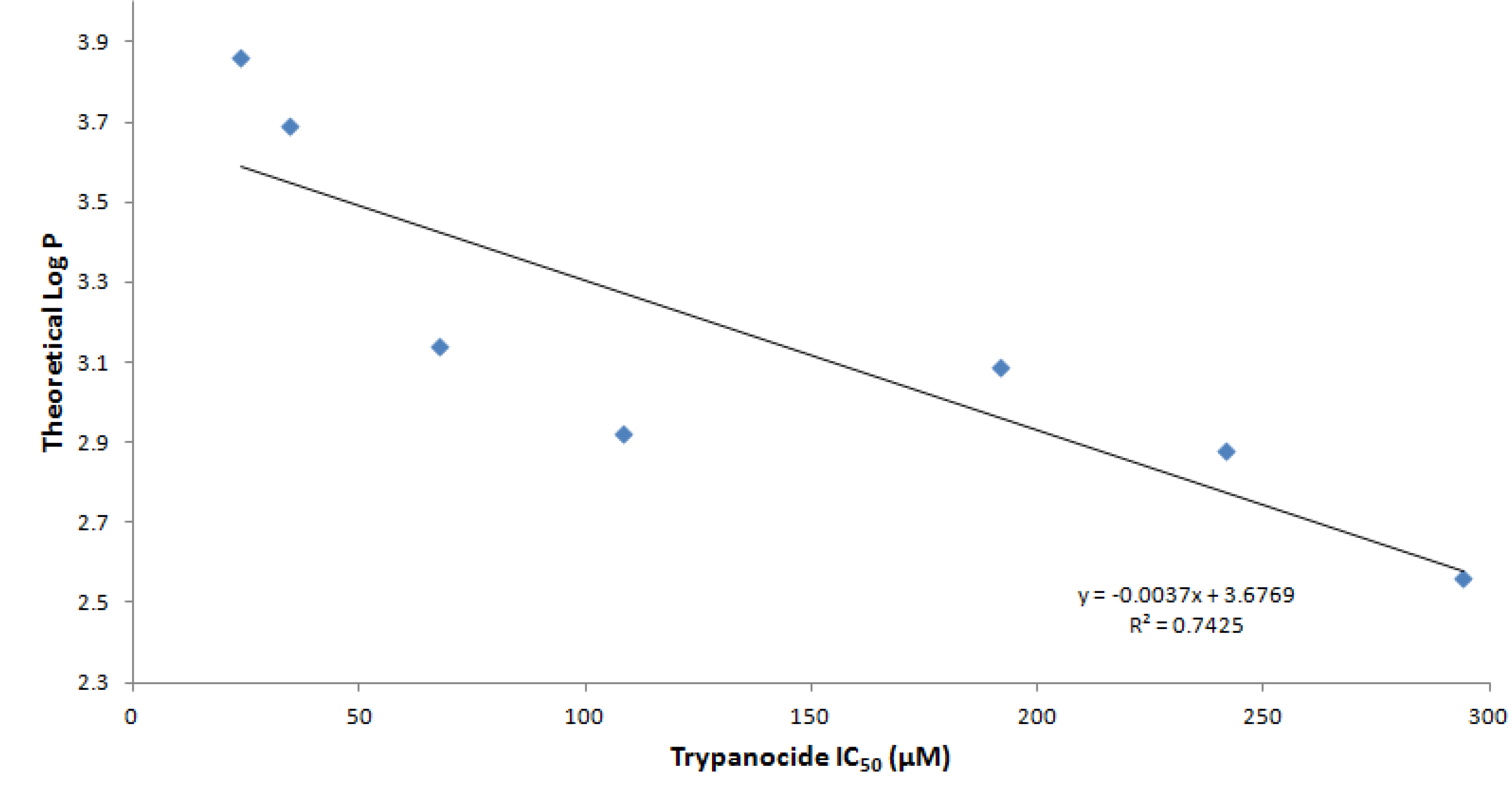
2.2. Trypanocidal Activity

| Cpn | R1 | R2 | R3 | Molecular Formula | M.W. | Yield (%) | M.P. (°C) | IC50 (µM) a | log P values b |
|---|---|---|---|---|---|---|---|---|---|
| 3 | H | H | H | C19H15N5O3 | 361.35 | 69 | 217–218 | 108.2 ± 5.65 | 2.92 ± 0.98 |
| 4 | H | F | H | C19H14FN5O3 | 379.38 | 48 | 248–249 | 67.8 ± 8.39 | 3.14 ± 1.02 |
| 5 | H | Cl | H | C19H14ClN5O3 | 395.80 | 20 | 249–250 | 34.6 ± 2.43 | 3.69 ± 0.99 |
| 6 | H | Br | H | C19H14BrN5O3 | 440.25 | 69 | 218–219 | 23.9 ± 4.88 | 3.86 ± 1.02 |
| 7 | H | NO2 | H | C19H14N6O5 | 406.35 | 62 | 361–362 | 241.6 ± 37.6 | 2.88 ± 0.99 |
| 8 | H | OCH3 | H | C20H17N5O4 | 391.38 | 62 | 218–219 | 191.8 ± 25.3 | 3.09 ± 0.99 |
| 9 | H | OH | H | C19H15N5O4 | 377.35 | 71 | 145–146 | 294.1 ± 27.98 | 2.56 ± 0.99 |
| 10 | OH | OH | H | C20H15N5O5 | 393.35 | 42 | 224–225 | 360.5 ± 24.67 | 2.34 ± 0.99 |
| 11 | OH | OCH3 | H | C20H17FN5O5 | 407.38 | 45 | 213–214 | 199.9 ± 2.02 | 2.53 ± 1.00 |
| 12 | OCH3 | OH | NO2 | C20H16N6O7 | 452.38 | 43 | 245–247 | 241.8 ± 7.54 | 3.22 ± 1.01 |
| Bzn | - | - | - | - | - | - | - | 10.8 ± 0.4 | 0.91 ± 1.00 |
| TAI Compound | % Cell Viability/concentration (μM) | ||
|---|---|---|---|
| 100 | 10 | 1 | |
| 4 | 97.6 | 98.9 | 94.2 |
| 5 | 93.1 | 97.6 | 95.7 |
| 6 | 78.7 | 97.6 | 97.3 |
3. Experimental
3.1. General Procedures
3.2. Procedure for the Synthesis of (Z)-2-(1-Methyl-5-nitro-1H-imidazol-2-yl)-1-phenylvinyl benzoate (14) [26]
3.3. Procedure for the Synthesis of (1E)-1-(1-Methyl-5-nitro-1H-imidazol-2-yl)-2-phenylethane-1,2-dione 1-oxime (15) [26]
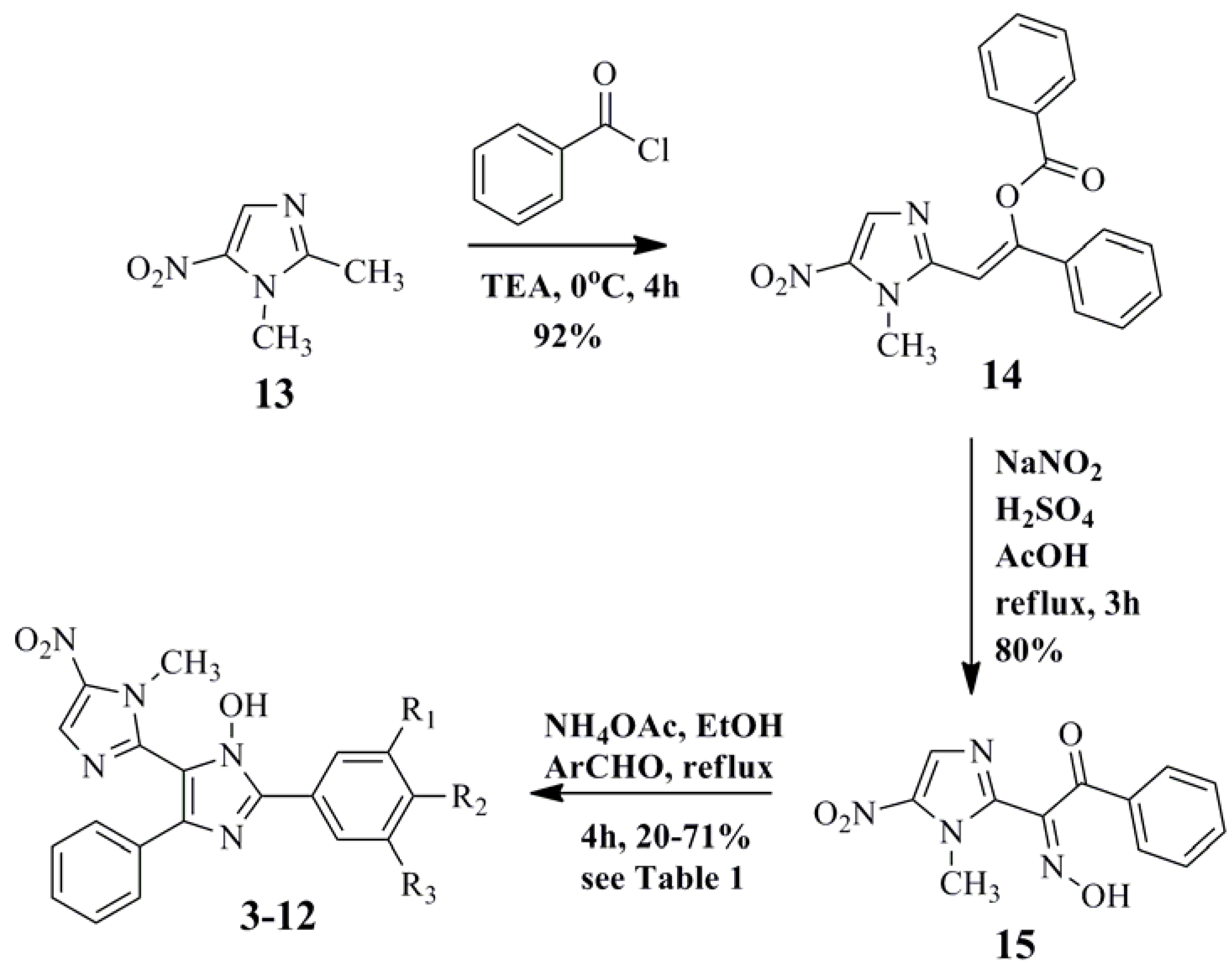
3.4. General Procedure for the Synthesis of 2,4,5-Trisubstituted Imidazole Derivatives (3–12) [27]
3.5. Activity against Bloodstream Trypomastigote Forms [28]
3.6. Cytotoxic Effect on Murine Macrophages
4. Conclusions
Acknowledgments
References
- World Health Organization (WHO), Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases; WHO: Geneva, Switzerland, 2010; pp. 1–172.
- Rassi-Jr, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Ministério da Saúde do Brasil. Secretaria Nacional de Vigilância em Saúde. Doença de Chagas Aguda. Manual Prático de Subsídio à Notificação Obrigatória no SINAN. Available online: http://portal.saude.gov.br/portal/arquivos/pdf/manual_chagas.pdf (accessed on 10 June 2012).
- Rassi-Jr, A.; Rassi, A.; Marcondes de Rezende, J. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- de Castro, S.L.; de Meirelles, M.N. Effect of drugs on Trypanosoma cruzi and on its interaction with heart muscle cell “in vitro”. Mem. Inst. Oswaldo Cruz 1987, 82, 209–218. [Google Scholar]
- Filard, L.S.; Brener, Z. A nitroimidazole-thiadiazole derivative with curative action in experimental Trypanosoma cruzi infections. Ann. Trop. Med. Parasitol. 1982, 76, 293–297. [Google Scholar]
- Lages-Silva, E.; Filard, L.S.; Brener, Z. Effect of the host specific treatment in the phagocytosis of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Mem. Inst. Oswaldo Cruz 1990, 85, 401–405. [Google Scholar] [CrossRef]
- Maya, J.D.; Bollo, S.; Nunez-Vergara, L.J.; Squella, J.A.; Repetto, Y.; Morello, A.; Périé, J.; Chauviére, G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003, 65, 999–1006. [Google Scholar] [CrossRef]
- Viodé, C.; Bettache, N.; Cenas, N.; Krauth-Siegel, R.L.; Chauvaviére, G.; Bakalara, N.; Périé, J. Enzymatic reduction studies of nitroheterocycles. Biochem. Pharmacol. 1999, 57, 549–557. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Ferreira, L.C. Mutagenicity of CL 64855, a potent anti-Trypanosoma cruzi drug. Mut. Res. 1986, 171, 11–15. [Google Scholar] [CrossRef]
- Nesslany, F.; Brugier, S.; Mouriès, M.A.; Le Curieux, F.; Marzin, D. In vitro and in vivo in vivo chromosomal aberrations induced by megazol. Mut. Res. 2004, 560, 147–158. [Google Scholar] [CrossRef]
- Poli, P.; Mello, M.A.; Buschini, A.; Mortara, R.A.; Albuquerque, C.; Silva, S.; Rossi, C.; Zucchi, T.M. Cytotoxic and genotoxic effects of megazol, an anti-Chagas' disease drug, Assessed by different short-term tests. Biochem. Pharmacol. 2002, 64, 1617–1627. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, A.S.; Fernandez-Ferreira, E.; Soares, R.O.; Souza, A.S.; Gibaldi, D.; Bozza, M.; Pinto, A.C. Novel nitroimidazoles with trypanocidal and cell growth inhibition activities. Cytobios 2001, 105, 83–90. [Google Scholar]
- Carvalho, S.A.; da Silva, E.F.; Santa-Rita, R.M.; de Castro, S.L.; Fraga, C.A.M. Synthesis and antitrypanosomal profile of new functionalized 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg. Med. Chem. Lett. 2004, 14, 5967–5970. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Lopes, F.A.S.; Salomão, K.; Romeiro, N.C.; Wardell, S.M.V.S.; de Castro, S.L.; da Silva, E.F.; Fraga, C.A.M. Studies toward the structural optimization of new brazilizone-relatedtrypanocidal 1,3,4-thiadiazole-2-arylhydrazone derivatives. Bioorg. Med. Chem. 2008, 16, 413–421. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Menna-Barreto, R.F.S.; Romeiro, N.C.; de Castro, S.L.; Boechat, N. Design, synthesis and activity against Trypanosoma cruzi of azaheterocyclic analogs of megazol. Med. Chem. 2007, 3, 460–465. [Google Scholar] [CrossRef]
- Salomão, K.; de Souza, E.M.; Carvalho, S.A.; da Silva, E.F.; Fraga, C.A.M.; Barbosa, H.S.; de Castro, S.L. In vitro and in vivo activities of 1,3,4-thiadiazole-2-arylhydrazone derivatives of megazol against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2010, 54, 2023–2031. [Google Scholar] [CrossRef]
- Katritzky, A.R. Comprehensive Heterocyclic Chemistry; Pergamon: Exeter, UK, 1984; Volume V, pp. 469–498. [Google Scholar]
- Mukherjee, A.; Kumar, S.; Seth, M.; Bhaduri, A.P. Synthesis of 1-methyl-4-nitro-5-substituted imidazole and substituted imidazolothiazole derivatives as possible antiparasitic agents. Indian J. Chem. 1989, 28B, 391–396. [Google Scholar]
- AyhanKilcigil, G.; Altanlar, N. Synthesis and antifungal properties of some benzimidazole derivatives. Turk. J. Chem. 2006, 30, 223–228. [Google Scholar]
- Norman, S.M.; Bennett, R.D.; Poling, S.M.; Maier, V.P.; Nelson, M.D. Paclobutrazol inhibits abscisic acid biosynthesis in Cercospora rosicola. Plant Physiol. 1986, 80, 122–125. [Google Scholar] [CrossRef]
- Guven, O.O.; Erdogan, T.; Goker, H.; Yildiz, S. Synthesis and antimicrobial activity of some novelphenyl and benzimidazole substituted benzyl ethers. Bioorg. Med. Chem. Lett. 2007, 17, 2233–2236. [Google Scholar]
- Hadizadeh, F.; Hosseinzadeh, H.; Motamed-Shariaty, V.-S.; Seifi, M.; Kazemi, S. Synthesis and antidepressant activity of N-substituted imidazole-5-carboxamides in forced swimming test model. Iranian J. Pharm. Res. 2008, 7, 29–33. [Google Scholar]
- Franchetti, P.; Stein, M.L.; Grifantini, M.; Lucarelli, C. Structure-activity relationships in reactivators of acetylcholinesterase inhibited by organophosphorus compounds. 4. N-hydroxyimidazole 3-oxides and their quaternary salts. Farmaco-Ed Sci. 1972, 27, 46–59. [Google Scholar]
- Viegas-Jr, C.; Danuello, A.; Bolzani, V.B.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Albright, J.D.; Shepherd, R.G. 1,2-disubstituted-5-nitroimidazoles. US Patent n 3, 652,555, 1972. [Google Scholar]
- Wang, M.; Gao, J.; Song, Z. A practical and green approach toward synthesis of 2,4,5-trisubstituted imidazoles without adding catalyst. Prep. Biochem. Biotechnol. 2010, 40, 347–353. [Google Scholar] [CrossRef]
- Salomão, K.; Dantas, A.P.; Borba, C.M.; Campos, L.C.; Machado, D.G.; Aquino-Neto, F.R.; de Castro, S.L. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Applied Microbiol. 2004, 38, 87–92. [Google Scholar] [CrossRef]
- de Castro, S.L.; Pinto, M.C.; Pinto, A.V. Screening of natural and synthetic drugs against Trypanosoma cruzi: I — Establishing a structure/activity relationship. Microbios 1994, 78, 83–90. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Da Silva, R.B.; Loback, V.B.; Salomão, K.; De Castro, S.L.; Wardell, J.L.; Wardell, S.M.S.V.; Costa, T.E.M.M.; Penido, C.; De Henriques, M.D.G.M. O.; Carvalho, S.A.; et al. Article Synthesis and Trypanocidal Activity of Novel 2,4,5-Triaryl-N-Hydroxylimidazole Derivatives. Molecules 2013, 18, 3445-3457. https://doi.org/10.3390/molecules18033445
Da Silva RB, Loback VB, Salomão K, De Castro SL, Wardell JL, Wardell SMSV, Costa TEMM, Penido C, De Henriques MDGMO, Carvalho SA, et al. Article Synthesis and Trypanocidal Activity of Novel 2,4,5-Triaryl-N-Hydroxylimidazole Derivatives. Molecules. 2013; 18(3):3445-3457. https://doi.org/10.3390/molecules18033445
Chicago/Turabian StyleDa Silva, Ramon Borges, Vanessa Brandão Loback, Kelly Salomão, Solange Lisboa De Castro, James L. Wardell, Solange M. S. V. Wardell, Thadeu Estevam Moreira Maramaldo Costa, Carmen Penido, Maria Das Graças Muller Oliveira De Henriques, Samir Aquino Carvalho, and et al. 2013. "Article Synthesis and Trypanocidal Activity of Novel 2,4,5-Triaryl-N-Hydroxylimidazole Derivatives" Molecules 18, no. 3: 3445-3457. https://doi.org/10.3390/molecules18033445






