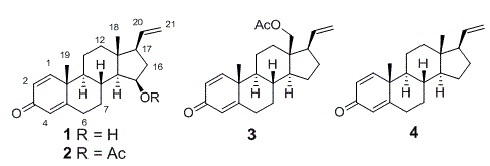
Bioactive Pregnane Steroids from a South China Sea Gorgonian Carijoa sp.
Abstract
:1. Introduction
2. Results and Discussion
| Positon | δH | δC | HMBC |
|---|---|---|---|
| 1 | 7.21 d (10.1) | 156.9 | C-3, C-5, C-9, C-10, C-19 |
| 2 | 6.11 dd (10.1, 1.9) | 127.1 | C-10 |
| 3 | – | 185.3 | |
| 4 | 6.00 s | 123.3 | C-6, C-10 |
| 5 | – | 170.1 | |
| 6 | 2.48 m2.33 m | 32.4 | C-5 |
| 7 | 2.27 m0.98 m | 32.7 | |
| 8 | 1.94 m | 31.7 | |
| 9 | 1.05 m | 53.1 | C-19 |
| 10 | – | 43.8 | |
| 11 | 1.68 m1.61 m | 22.3 | |
| 12 | 1.57 m1.01 m | 38.3 | C-18 |
| 13 | – | 43.3 | |
| 14 | 0.78 dd (11.1, 5.8) | 59.0 | C-8, C-13, C-18 |
| 15 | 4.10 m | 69.0 | |
| 16 | 2.18 m ( α ) | 39.7 | C-13 |
| 1.51 m ( β ) | |||
| 17 | 1.85 m | 55.0 | |
| 18 | 0.87 s | 15.7 | C-12, C-13, C-14, C-17 |
| 19 | 1.22 s | 18.9 | C-1, C-5, C-9, C-10 |
| 20 | 5.79 ddd(17.3, 10.4, 7.4) | 139.6 | |
| 21 | 4.98 dd (10.4, 1.7) | 115.3 | C-17 |
| 4.96 dd (17.3, 1.7) | |||
| 15-OH | 4.45 d (4.0) |


| Strains | Compounds | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Ciprofloxacin | |
| B. cereus | >64.0 | 5.00 | 2.50 | >50.0 | 0.078 |
| S. aureus | 0.063 | >50.0 | 0.156 | >50.0 | 0.019 |
| S. albus | 1.00 | 2.50 | >50.0 | >50.0 | 0.312 |
| T. halophilus | >64 | 0.312 | 1.25 | >50.0 | 0.019 |
| E. coli | 1.00 | 2.50 | >50.0 | >50.0 | 0.625 |
| P. putida | 0.031 | 1.25 | >50.0 | >50.0 | 0.156 |
| V. parahaemolyticus | 4.00 | 50.0 | >50.0 | >50.0 | 2.50 |
| N. brasiliensis | 0.500 | 1.25 | >50.0 | >50.0 | 0.078 |
3. Experimental
3.1. General Procedures
3.2. Animal Materials
3.3. Extraction and Isolation
3.4. Hydrolysis of Compound 2
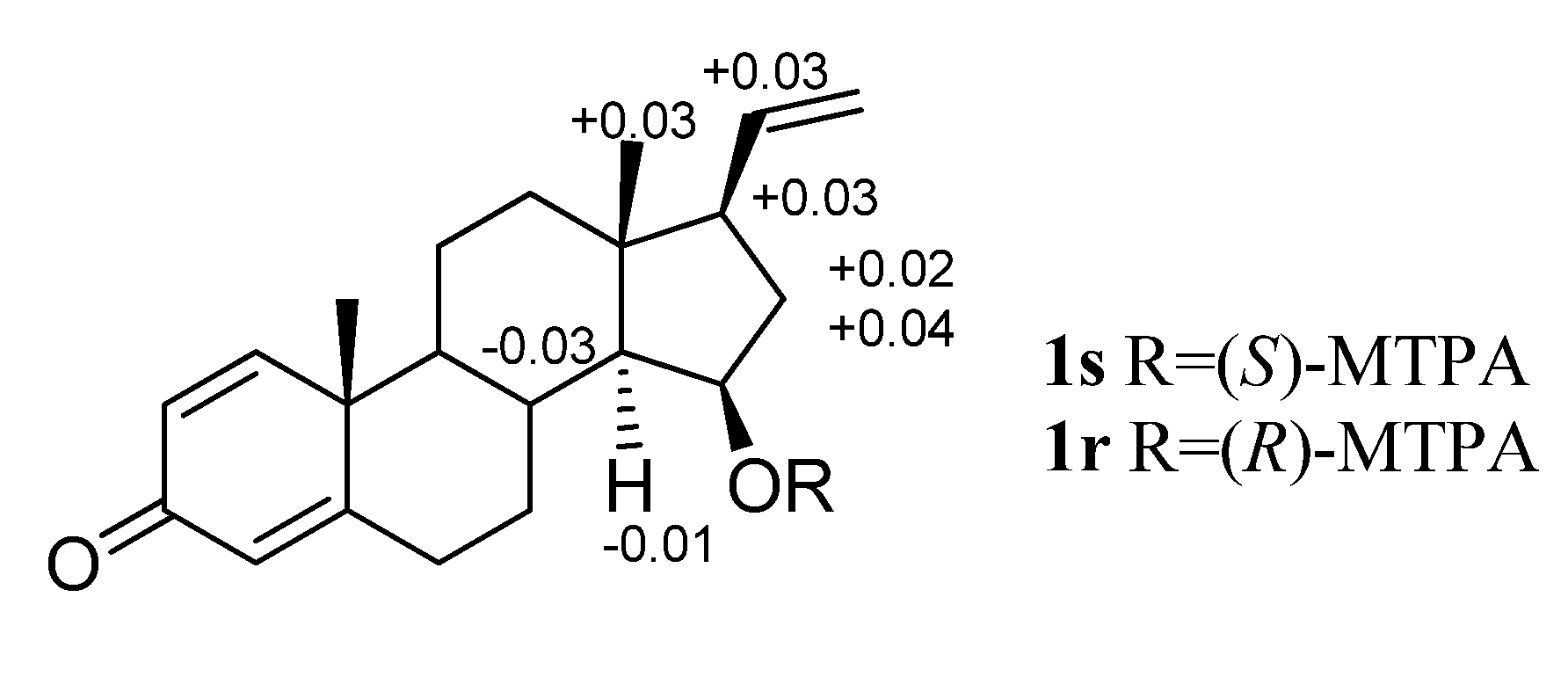
3.5. Preparation of the (S)- and (R)-MTPA Ester Derivatives of Compound 1
3.6. Brine Shrimp Lethality Assay
3.7. Cytotoxicity Test
3.8. Antibacterial Activity Assay
4. Conclusions
Acknowledgments
Supplementary Materials
References
- Poza, J.J.; Fernández, R.; Reyes, F.; Rodríguez, J.; Jiménez, C. Isolation, biological significance, synthesis, and cytotoxic evaluation of new natural parathiosteroids A–C and analogues from the soft coral Paragorgia sp. J. Org. Chem. 2008, 73, 7978–7984. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Porras, G.; Aragón, Z.; de la Rosa, J.M.; Dorta, E.; Cueto, M.; D’Croz, L.; Maté, J.; Darias, J. Carijodienone from the octocoral Carijoa multiflora. a spiropregnane-based steroid. J. Nat. Prod. 2011, 74, 292–295. [Google Scholar] [CrossRef]
- Wu, S.L.; Wang, G.H.; Dai, C.F.; Sheu, J.H. Pregnane-based steroids from a Formosan gorgonian Subergorgia mollis. J. Chin. Chem. Soc. 2004, 51, 205–208. [Google Scholar]
- Reimão, J.Q.; Migotto, A.E.; Kossuga, M.H.; Berlinck, R.G.S.; Tempone, A.G. Antiprotozoan activity of Brazilian marine cnidarian extracts and of a modified steroid from the octocoral Carijoa riisei. Parasitol Res. 2008, 103, 1445–1450. [Google Scholar] [CrossRef]
- Regalado, E.L.; Tasdemir, D.; Kaiser, M.; Cachet, N.; Amade, P.; Thomas, O.P. Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J. Nat. Prod. 2010, 73, 1404–1410. [Google Scholar] [CrossRef]
- Chao, C.H.; Wen, Z.H.; Su, J.H.; Chen, I.M.; Huang, H.C.; Dai, C.F.; Sheu, J.H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffin. Steroids 2008, 73, 1353–1358. [Google Scholar] [CrossRef]
- Fang, H.Y.; Liaw, C.C.; Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Hsu, C.H.; Dai, C.F.; Sheu, J.H. Bioactive pregnane-type steroids from the soft coral Scleronephthya gracillimum. Tetrahedron 2012, 68, 9694–9700. [Google Scholar] [CrossRef]
- Wang, S.K.; Dai, C.F.; Duh, C.Y. Cytotoxic pregnane steroids from the Formosan soft coral Stereonephthya crystalliana. J. Nat. Prod. 2006, 69, 103–106. [Google Scholar] [CrossRef]
- Han, L.; Wang, C.Y.; Huang, H.; Shao, C.L.; Liu, Q.A.; Qi, J.; Sun, X.P.; Zhai, P.; Gu, Y.C. A new pregnane analogue from Hainan soft coral Scleronephthya gracillimum Kuekenthal. Biochem. Sys. Ecol. 2010, 38, 243–246. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhao, J.; Liu, H.Y.; Shao, C.L.; Liu, Q.A.; Liu, Y.; Gu, Y.C. Two new eicosanoids with a unique isovalerianic acid ester moiety from the South China Sea gorgonian Dichotella gemmacea. Lipids 2011, 46, 81–85. [Google Scholar] [CrossRef]
- Li, R.; Shao, C.L.; Qi, X.; Li, X.B.; Li, J.; Sun, L.L.; Wang, C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef]
- Li, L.; Sheng, L.; Wang, C.Y.; Zhou, Y.B.; Huang, H.; Li, X.B.; Li, J.; Mollo, E.; Gavagnin, M.; Guo, Y.W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011, 74, 2089–2094. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gresa, M.P.L.; Manzo, E.; Gavagnin, M.; Wahidulla, S.; Cimino, G. New C21Δ20 pregnanes, inhibitors of mitochondrial respiratory chain, from Indopacific octocoral Carijoa sp. Tetrahedron Lett. 2004, 45, 7745–7748. [Google Scholar]
- Kingston, J.F.; Gregory, B.; Fallis, A.G. Pregna-1,4,20-triene-3-one, a novel marine steroid from the sea raspberry Gersemia rubiformis. Tetrahedron Lett. 1977, 49, 4261–4264. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. 5α-pregna-1,20-dien-3-one and related compounds from a soft coral. Steroids 1977, 30, 379–388. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusuni, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Appendio, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis satiνa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Motta, A. Cytochalasins Z4, Z5, and Z6, three new 24-oxa[14] cytochalasans produced by Phoma exigua var. heteromorpha. J. Nat. Prod. 2003, 66, 1540–1544. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobson, L.B.; Nicols, D.E.; Mclaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Capson, T.; Guzmán, H.M.; Quiñoá, E.; Riguera, R. L-Galactose as a natural product: Isolation from a marine octocoral of the first α-L-galactosyl saponin. Tetrahedron. Lett. 2004, 45, 7833–7836. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Capson, T.L.; Guzmán, H.M.; González, J.; Ortega-Barría, E.; Quiñoá, E.; Riguera, R. Antiplasmodial metabolites isolated from the marine octocoral Muricea austere. J. Nat. Prod. 2006, 69, 1379–1383. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron 2008, 64, 11797–11801. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 3, 4 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, H.-Y.; Shao, C.-L.; Li, Z.-Y.; Han, L.; Cao, F.; Wang, C.-Y. Bioactive Pregnane Steroids from a South China Sea Gorgonian Carijoa sp. Molecules 2013, 18, 3458-3466. https://doi.org/10.3390/molecules18033458
Zhao H-Y, Shao C-L, Li Z-Y, Han L, Cao F, Wang C-Y. Bioactive Pregnane Steroids from a South China Sea Gorgonian Carijoa sp. Molecules. 2013; 18(3):3458-3466. https://doi.org/10.3390/molecules18033458
Chicago/Turabian StyleZhao, Hong-Ying, Chang-Lun Shao, Zhi-Yong Li, Lei Han, Fei Cao, and Chang-Yun Wang. 2013. "Bioactive Pregnane Steroids from a South China Sea Gorgonian Carijoa sp." Molecules 18, no. 3: 3458-3466. https://doi.org/10.3390/molecules18033458