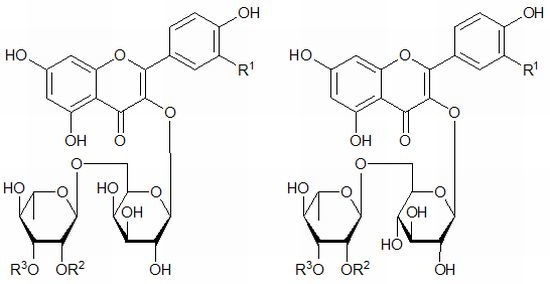
Rare Syringyl Acylated Flavonol Glycosides from the Aerial Parts of Leonurus japonicus Houtt.
Abstract
:1. Introduction
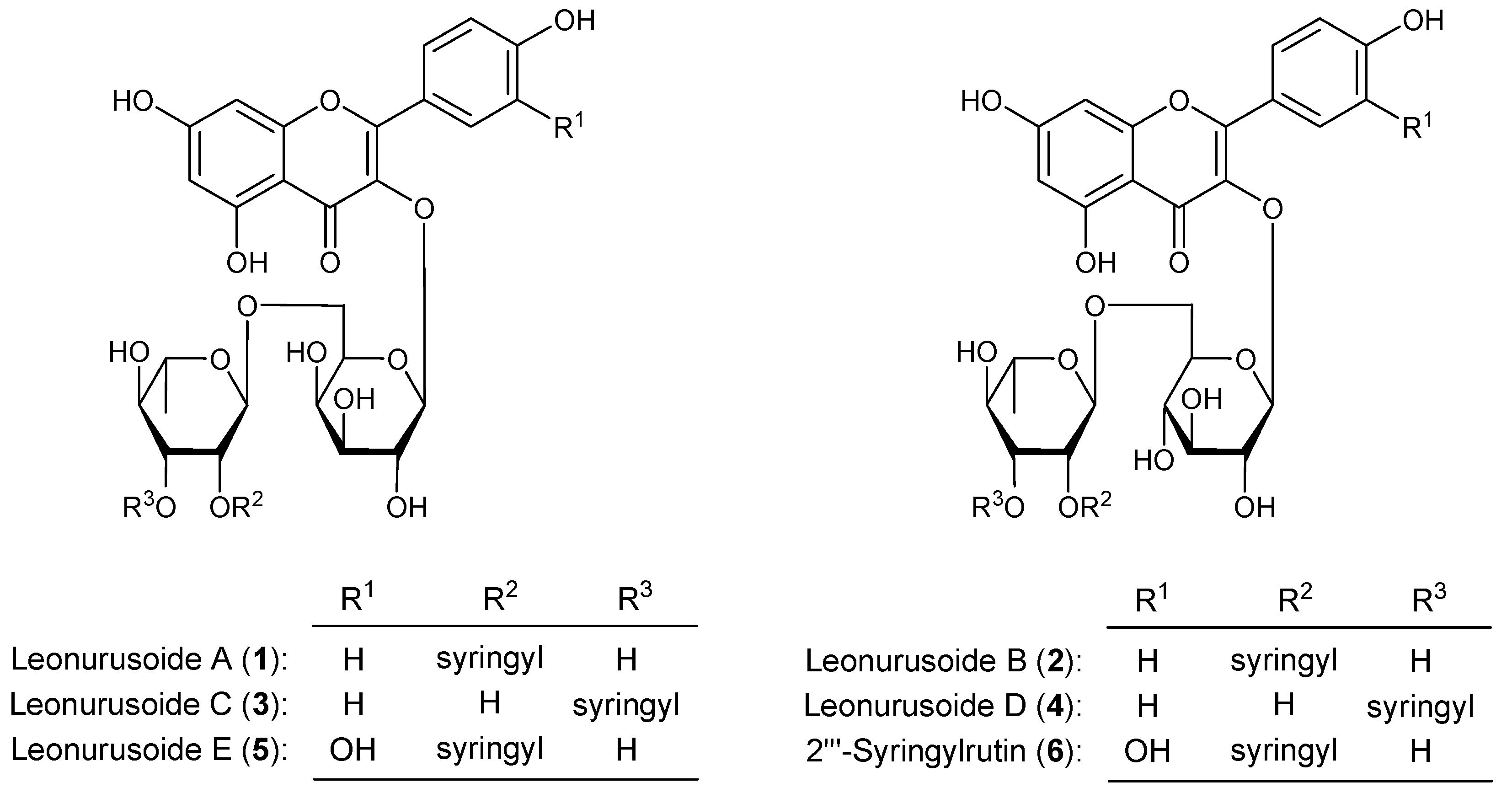
2. Results and Discussion
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 156.2 | — | 156.3 | — |
| 3 | 133.2 | — | 133.1 | — |
| 4 | 177.0 | — | 177.1 | — |
| 5 | 161.0 | — | 161.0 | — |
| 6 | 99.1 | 6.14 (1H, br. s) | 98.9 | 6.16 (1H, br. s) |
| 7 | 165.9 | — | 165.1 | — |
| 8 | 93.9 | 6.34 (1H, br. s) | 93.8 | 6.34 (1H, br. s) |
| 9 | 156.5 | — | 156.5 | — |
| 10 | 103.2 | — | 103.5 | — |
| 1' | 120.8 | — | 120.8 | — |
| 2',6' | 130.7 | 8.02 (2H, d, 8.5) | 130.7 | 7.96 (2H, d, 8.5) |
| 3',5' | 114.9 | 6.81 (2H, d, 8.5) | 114.9 | 6.83 (2H, d, 8.5) |
| 4' | 159.8 | — | 159.8 | — |
| 1'' | 102.2 | 5.30 (1H, d, 7.5) | 101.5 | 5.32 (1H, d, 7.5) |
| 2'' | 71.0 | 3.57 (1H, dd, 7.5, 9.0) | 74.1 | 3.18 (1H, dd, 7.5, 9.0) |
| 3'' | 72.9 | 3.43 (1H, dd, 3.0, 9.0) | 76.3 | 3.23 (1H, dd, 9.0, 9.0) |
| 4'' | 67.9 | 3.65 (1H, m, overlapped) | 69.7 | 3.08 (1H, dd, 9.0, 9.0) |
| 5'' | 73.2 | 3.63 (1H, m, overlapped) | 75.6 | 3.29 (1H, m) |
| 6'' | 65.3 | 3.32 (1H, dd, 5.0, 9.0) | 66.8 | 3.38 (1H, dd, 5.0, 9.0) |
| 3.66 (1H, m, overlapped) | 3.73 (1H, br. d, ca. 11) | |||
| 5-OH | 12.53 (1H, br. s) | 12.54 (1H, br. s) | ||
| 1''' | 96.9 | 4.57 (1H, br. s) | 97.6 | 4.53 (1H, br. s) |
| 2''' | 72.5 | 4.92 (1H, br. d, ca. 3) | 72.5 | 4.95 (1H, br. d, ca. 3) |
| 3''' | 68.4 | 3.63 (1H, m, overlapped) | 68.6 | 3.61 (1H, dd, 3.0, 9.0) |
| 4''' | 72.4 | 3.26 (1H, dd, 9.5, 9.5) | 72.4 | 3.24 (1H, dd, 9.0, 9.5) |
| 5''' | 68.6 | 3.52 (1H, m) | 68.4 | 3.41 (1H, m) |
| 6''' | 18.0 | 1.15 (3H, d, 6.0) | 17.8 | 1.07 (3H, d, 6.0) |
| 1'''' | 119.2 | — | 119.4 | — |
| 2'''',6'''' | 107.0 | 7.19 (2H, s) | 107.1 | 7.19 (2H, s) |
| 3'''',5'''' | 147.4 | — | 147.4 | — |
| 4'''' | 140.9 | — | 140.7 | — |
| 7'''' | 164.7 | — | 164.7 | — |
| -OCH3 | 55.9 | 3.81 (6H, s) | 56.0 | 3.82 (6H, s) |

| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 156.4 | — | 156.7 | — |
| 3 | 133.2 | — | 133.2 | — |
| 4 | 177.3 | — | 177.2 | — |
| 5 | 161.1 | — | 161.1 | — |
| 6 | 98.7 | 6.18 (1H, br. s) | 98.7 | 6.16 (1H, d, 1.5) |
| 7 | 164.3 | — | 164.3 | — |
| 8 | 93.7 | 6.42 (1H, br. s) | 93.7 | 6.38 (1H, d, 1.5) |
| 9 | 156.3 | — | 156.4 | — |
| 10 | 103.7 | — | 103.8 | — |
| 1' | 120.7 | — | 120.8 | — |
| 2',6' | 130.9 | 8.06 (2H, d, 8.0) | 130.7 | 7.98 (2H, d, 8.5) |
| 3',5' | 115.0 | 6.87 (2H, d, 8.0) | 115.0 | 6.88 (2H, d, 8.5) |
| 4' | 159.9 | — | 159.8 | — |
| 1'' | 102.0 | 5.34 (1H, d, 8.0) | 101.5 | 5.32 (1H, d, 7.5) |
| 2'' | 71.0 | 3.57 (1H, dd, 8.0, 9.0) | 74.1 | 3.19 (1H, dd, 7.5, 8.5) |
| 3'' | 72.8 | 3.43 (1H, dd, 3.0, 9.0) | 76.3 | 3.24 (1H, dd, 8.5, 8.5) |
| 4'' | 67.7 | 3.67 (1H, m, overlapped) | 69.9 | 3.07 (1H, dd, 8.5, 9.0) |
| 5'' | 73.1 | 3.64 (1H, m, overlapped) | 75.6 | 3.33 (1H, m) |
| 6'' | 65.0 | 3.29 (1H, dd, 5.0, 9.0) | 67.3 | 3.37 (1H, dd, 5.5, 12.0) |
| 3.67 (1H, m, overlapped) | 3.75 (1H, dd, 2.0, 12.0) | |||
| 5-OH | 12.57 (1H, br. s) | 12.51 (1H, br. s) | ||
| 1''' | 99.9 | 4.51 (1H, br. s) | 100.8 | 4.46 (1H, d, 1.5) |
| 2''' | 68.0 | 3.75 (1H, br. d, ca. 3) | 67.8 | 3.77 (1H, dd, 1.5, 3.5) |
| 3''' | 74.7 | 4.84 (1H, dd, 3.0, 9.5) | 74.7 | 4.78 (1H, dd, 3.5, 9.5) |
| 4''' | 68.9 | 3.49 (1H, dd, 9.5, 9.5) | 68.8 | 3.49 (1H, dd, 8.5, 9.5) |
| 5''' | 68.3 | 3.58 (1H, m) | 68.3 | 3.47 (1H, m) |
| 6''' | 17.8 | 1.14 (3H, d, 6.0) | 17.6 | 1.11 (3H, d, 6.0) |
| 1'''' | 119.8 | — | 119.9 | — |
| 2'''',6'''' | 107.2 | 7.27 (2H, s) | 107.2 | 7.28 (2H, s) |
| 3'''',5'''' | 147.3 | — | 147.3 | — |
| 4'''' | 140.4 | — | 140.4 | — |
| 7'''' | 165.3 | — | 165.2 | — |
| -OCH3 | 56.0 | 3.81 (6H, s) | 56.0 | 3.82 (6H, s) |
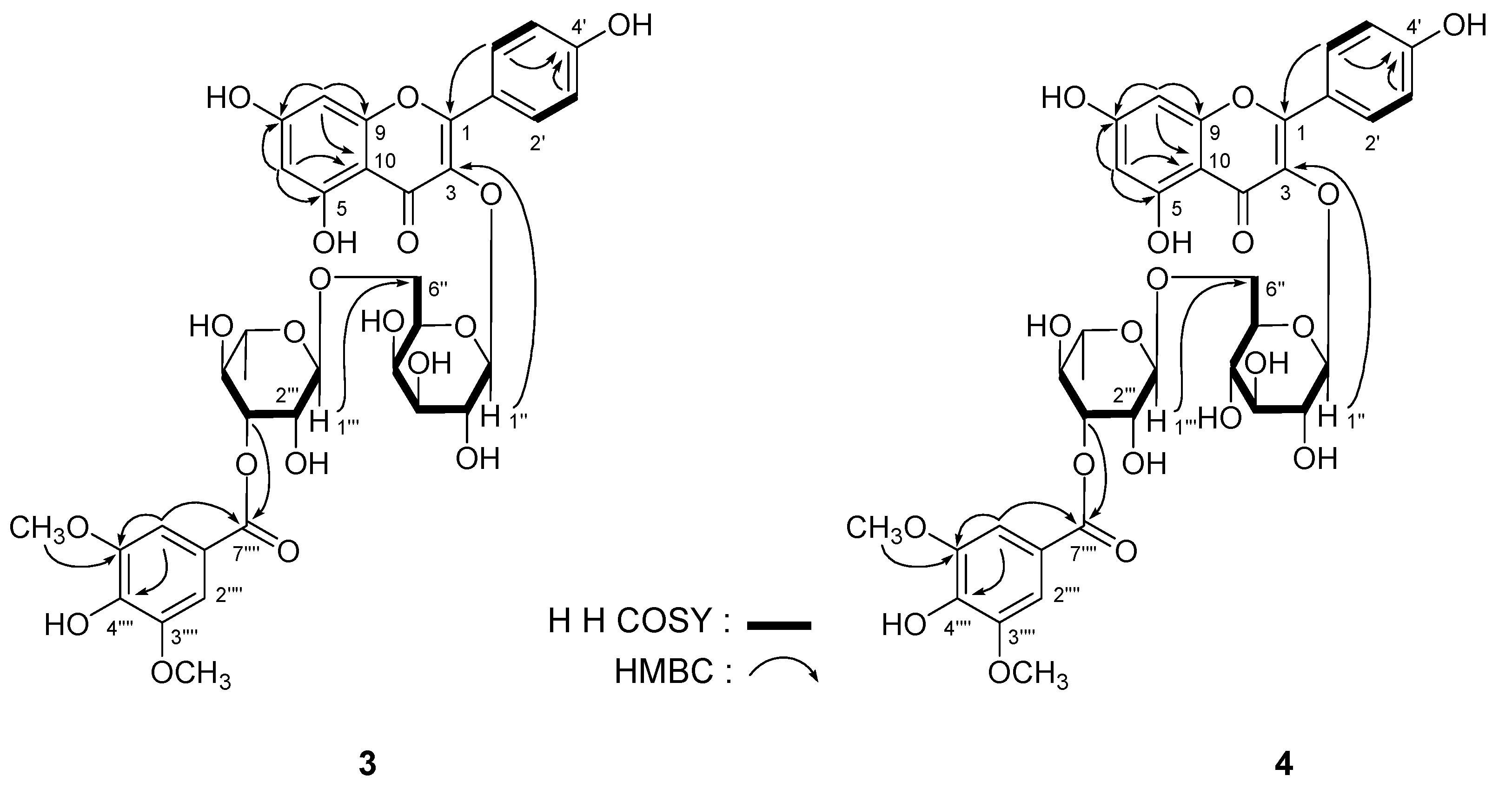
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
|---|---|---|---|---|---|
| 2 | 156.2 | — | 3'' | 72.9 | 3.43 (1H, dd, 3.0, 9.5) |
| 3 | 133.4 | — | 4'' | 67.8 | 3.68 (1H, m, overlapped) |
| 4 | 177.2 | — | 5'' | 73.2 | 3.64 (1H, m, overlapped) |
| 5 | 161.1 | — | 6'' | 65.1 | 3.33 (1H, dd, 5.5, 9.5) |
| 6 | 98.6 | 6.20 (1H, br. s) | 3.67 (1H, m, overlapped) | ||
| 7 | 164.3 | — | 1''' | 96.8 | 4.62 (1H, d, 1.0) |
| 8 | 93.5 | 6.39 (1H, br. s) | 2''' | 72.6 | 4.93 (1H, dd, 1.0, 3.0) |
| 9 | 156.2 | — | 3''' | 68.6 | 3.65 (1H, m, overlapped) |
| 10 | 103.7 | — | 4''' | 72.4 | 3.28 (1H, dd, 9.0, 9.5) |
| 1' | 121.0 | — | 5''' | 68.4 | 3.54 (1H, m) |
| 2' | 115.9 | 7.54 (1H, d, 2.0) | 6''' | 18.0 | 1.16 (3H, d, 6.0) |
| 3' | 144.7 | — | 1'''' | 119.2 | — |
| 4' | 148.4 | — | 2'''',6'''' | 107.0 | 7.20 (2H, s) |
| 5' | 115.0 | 6.80 (1H, d, 8.5) | 3'''', 5'''' | 147.4 | — |
| 6' | 121.7 | 7.63 (1H, d, 2.0, 8.5) | 4'''' | 140.7 | — |
| 5-OH | 12.58 (1H, br. s) | 7'''' | 164.7 | ||
| 1'' | 101.9 | 5.35 (1H, d, 8.0) | -OCH3 | 55.9 | 3.81 (6H, s) |
| 2'' | 71.0 | 3.60 (1H, dd, 8.0, 9.0) |

| Concentration ( μM) | Inhibitory rate (%) | |
|---|---|---|
| Control | 0 | 0.0 ± 1.2 |
| Orlistat | 1 | 43.3 ± 4.1 * |
| 1 | 10 | 61.4 ± 10.0 ** |
| 2 | 10 | 52.8 ± 4.2 * |
| 3 | 10 | 56.9 ± 9.6 ** |
| 4 | 10 | 51.7 ± 6.9 * |
| 5 | 10 | 49.1 ± 4.9 * |
| 6 | 10 | 44.3 ± 4.3 * |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
Acid Hydrolysis of 1–5
3.4. TG Accumulation Inhibitory Effects Assay
Statistical Analysis
4. Conclusions
Acknowledgments
References
- National Pharmacopoeia Committee, The People’s Republic of China Pharmacopoeia; Chinese medicine science and Technology Press: Beijing, China, 2010; p. 273.
- Giang, P.M.; Son, P.T.; Matsunami, K.; Otsuka, H. New labdane-type diterpenoids from Leonurus heterophyllus SW. In Chem. Pharm. Bull.; 2005; Volume 53, pp. 938–941. [Google Scholar]
- Giang, P.M.; Son, P.T.; Matsunami, K.; Otsuka, H. New bis-spirolabdane-type diterpenoids from Leonurus heterophyllus SW. In Chem. Pharm. Bull.; 2005; Volume 53, pp. 1475–1479. [Google Scholar]
- Cai, X.H.; Che, C.T.; Lam, C.K.; Mak, T.C.; Wu, L.J. A new labdane diterpene from Leonurus heterophyllus. J. Asian Nat. Prod. Res. 2006, 8, 599–603. [Google Scholar] [CrossRef]
- Romero-González, R.R.; Avila-Núñez, J.L.; Aubert, L.; Alonso-Amelot, M.E. Labdane diterpenes from Leonurus japonicus leaves. Phytochemistry 2006, 67, 965–970. [Google Scholar] [CrossRef]
- Seo, H.K.; Kim, J.S.; Kang, S.S. Labdane diterpenes and flavonoids from Leonurus japonicus. Helvetica Chimica Acta 2010, 93, 2045–2051. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, J.H.; Li, X. A new flavonoside from Leonurus heterophyllus. J. Asian Nat. Prod. Res. 2005, 7, 273–277. [Google Scholar] [CrossRef]
- Cai, X.; Che, Z.; Wu, B.; Gao, H.; Wu, L. Studies on chemical constituents of Leonurus japonicus. Shenyang Yaoke Daxue Xuebao 2006, 23, 13–21. [Google Scholar]
- Cong, Y.; Guo, J.; Wang, X.; Li, M.; Li, K.; Wang, J.; Li, Q. Chemical constituents and antitumor activity on leukem ia K562 cell of Leonurus heterophyllus. Zhongguo Zhong Yao Za Zhi 2009, 34, 1816–1818. [Google Scholar]
- Li, Y.; Chen, Z.; Feng, Z.; Yang, Y.; Jiang, J.; Zhang, P. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012, 348, 42–46. [Google Scholar] [CrossRef]
- Morikawa, T.; Zhang, Y.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXII. Absolute structures of new megastigmane glycosides, sedumosides E1, E2, E3, F1, F2, and G, from Sedum sarmentosum (Crassulaceae). Chem. Pharm. Bull. 2007, 55, 435–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Morikawa, T.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Muraoka, O.; Yoshikawa, M. Bioactive constituents from Chinese Natural Medicines. XXV. New flavonol bisdesmosides, sarmenosides I, II, III, and IV, with hepatoprotective activity from Sedum sarmentosum (Crassulaceae). Heterocycles 2007, 71, 1565–1576. [Google Scholar] [CrossRef]
- Williams, C.A. Flavonoids. In Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; p. 749. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 5 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Deng, S.; Qu, L.; An, Y.-T.; Wu, C.-H.; Han, L.-F.; Gao, X.-M.; Wang, T. Rare Syringyl Acylated Flavonol Glycosides from the Aerial Parts of Leonurus japonicus Houtt. Molecules 2013, 18, 2967-2977. https://doi.org/10.3390/molecules18032967
Zhang Y, Deng S, Qu L, An Y-T, Wu C-H, Han L-F, Gao X-M, Wang T. Rare Syringyl Acylated Flavonol Glycosides from the Aerial Parts of Leonurus japonicus Houtt. Molecules. 2013; 18(3):2967-2977. https://doi.org/10.3390/molecules18032967
Chicago/Turabian StyleZhang, Yi, Shen Deng, Lu Qu, Ya-Ting An, Chun-Hua Wu, Li-Feng Han, Xiu-Mei Gao, and Tao Wang. 2013. "Rare Syringyl Acylated Flavonol Glycosides from the Aerial Parts of Leonurus japonicus Houtt." Molecules 18, no. 3: 2967-2977. https://doi.org/10.3390/molecules18032967