Microwave-Enhanced Cross-Coupling Reactions Involving Alkynyltrifluoroborates with Aryl Bromides
Abstract
:1. Introduction
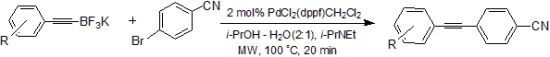
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. Coupling Reactions: General Procedure
5. Conclusions
Acknowledgments
References
- Brandsma, L.; Vasilevsky, S.F.; Vekruijsse, H.D. Application of Transition Metal Catalysis; Springer-Verlag: New York, NY, USA, 1998. [Google Scholar]
- Castro, C.E.; Stephens, R.D. The substitution of aryl iodides with cuprous acetylides. A synthesis of tolanes and heterocyclics. J. Org. Chem. 1963, 28, 3313–3315. [Google Scholar]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Sonogashira, K. Metal-Catalyzed Cross Coupling Reactions; Diederich, F., Stang, P.J., Eds.; Wiley-Interscience: New York, NY, USA, 2002; p. 493. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts: Innovations in Organic Synthesis; Wiley and Sons: Chichester, UK, 1997. [Google Scholar]
- King, A.O.; Okukado, N.; Negishi, E. Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides. J. Chem. Commun. 1977, 683–684. [Google Scholar] [CrossRef]
- Negishi, E.; Xu, C. Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., Ed.; Wiley: New York, NY, USA; pp. 531–549.
- Negishi, E.; Okukado, N.; King, A.O.; Van Horn, D.E.; Spiegel, B.I. Double metal catalysis in the cross-coupling reaction and its application to the stereo- and regioselective synthesis of trisubstituted olefins. J. Am. Chem. Soc. 1978, 100, 2254–2256. [Google Scholar] [CrossRef]
- Pelter, A.; Smith, K.; Brown, H.C. Borane Reagents; Academic Press: London, UK, 1998. [Google Scholar]
- De Mora, S.J. Tributyltin: Case Study of an Environmental Contaminant; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Boyer, I.J. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology 1989, 55, 253–298. [Google Scholar] [CrossRef]
- Pelter, A.; Smith, K.; Brown, H.C. Borane Reagents; Academic Press: London, UK, 1988. [Google Scholar]
- Molander, G.A.; Fleury-Bregeot, N.; Hiebel, M.-A. Synthesis and cross-coupling of sulfonamidomethyltrifluoroborates. Org. Lett. 2011, 13, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Molander, G.A.; Beaumard, F. Cross-coupling of mesylated phenol derivatives with potassium ammonio- and amidomethyltrifluoroborates. Org. Lett. 2011, 13, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Dadush, E.; Al-Masum, M. Microwave-enhanced cross-coupling of allyl chlorides with vinyltrifluoroborates. Tetrahedron Lett. 2006, 47, 7459–7461. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Al-Masum, M.; Mereddy, A.R.; Dadush, E. Microwave enhanced cross-coupling reactions involving alkenyl- and alkynyltrifluoroborates. Tetrahedron Lett. 2006, 47, 1133–1136. [Google Scholar] [CrossRef]
- Molander, G.A.; Katona, B.W.; Machrouhi, F. Development of the Suzuki-Miyaura cross-coupling reaction: Use of air-stable potassium alkynyltrifluoroborates in aryl alkynylations. J. Org. Chem. 2002, 67, 8416–8423. [Google Scholar] [CrossRef] [PubMed]
- Jouvin, K.; Couty, F.; Evano, G. Copper-catalyzed alkynylation of amides with potassium alkynyltrifluoroborates: A room-temperature, base-free synthesis of ynamides. Org. Lett. 2010, 12, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Ramondenc, Y.; Dupas, G.; Ple, N. Bis- and tris(arylethynyl)pyrimidine oligomers: Synthesis and light-emitting properties. Tetrahedron 2008, 64, 2783–2791. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Entry | R1BF3K | Ar-Br | Ar-R1 | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  |  | 75 |
| 2 |  |  |  | 65 |
| 3 |  |  |  | 60 |
| 4 |  |  |  | 54 |
| 5 |  |  |  | 40 |
| Entry | R1BF3K | Ar-Br | Ar-R1 | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  |  | 84 |
| 2 |  |  |  | 87 |
| 3 |  |  |  | 87 |
| 4 |  |  |  | 75 |
| 5 |  |  |  | 80 |
| 6 |  |  |  | 76 |
| 7 |  |  |  | 70 |
| 8 |  |  |  | 78 |
| 9 |  |  |  | 45 |
| 10 |  |  | NR |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coltuclu, V.; Dadush, E.; Naravane, A.; Kabalka, G.W. Microwave-Enhanced Cross-Coupling Reactions Involving Alkynyltrifluoroborates with Aryl Bromides. Molecules 2013, 18, 1755-1761. https://doi.org/10.3390/molecules18021755
Coltuclu V, Dadush E, Naravane A, Kabalka GW. Microwave-Enhanced Cross-Coupling Reactions Involving Alkynyltrifluoroborates with Aryl Bromides. Molecules. 2013; 18(2):1755-1761. https://doi.org/10.3390/molecules18021755
Chicago/Turabian StyleColtuclu, Vitali, Eric Dadush, Abhijit Naravane, and George W. Kabalka. 2013. "Microwave-Enhanced Cross-Coupling Reactions Involving Alkynyltrifluoroborates with Aryl Bromides" Molecules 18, no. 2: 1755-1761. https://doi.org/10.3390/molecules18021755