Antimalarial Activity of 4-Metoxychalcones: Docking Studies as Falcipain/Plasmepsin Inhibitors, ADMET and Lipophilic Efficiency Analysis to Identify a Putative Oral Lead Candidate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biological Activity
| Compound | IC50 (mM) | LC50 (mM) | SI |
|---|---|---|---|
| 1a | 2.06 ± 0.05 | 18.58 ± 1.26 | 9.0 |
| 1b | 5.79 ± 0.12 | 20.70 ± 1.62 | 3.6 |
| 1c | 2.03 ± 0.28 | 11.54 ± 0.23 | 5.7 |
| 1d | 3.64 ± 0.40 | 11.07 ± 1.96 | 3.0 |
| 1e | 2.83 ± 0.21 | 10.49 ± 2.27 | 3.7 |
| 1f | 4.33 ± 0.06 | 18.97 ± 0.43 | 4.4 |
| 1g | 10.99 ± 0.57 | 20.70 ± 1.60 | 1.9 |
| 1h | 1.96 ± 0.02 | 9.42 ± 2.09 | 4.8 |
| 1i | 2.07 ± 0.22 | 9.18 ± 1.51 | 4.4 |
| CQ a | 0.45 ± 0.04 | >100 | >100 |
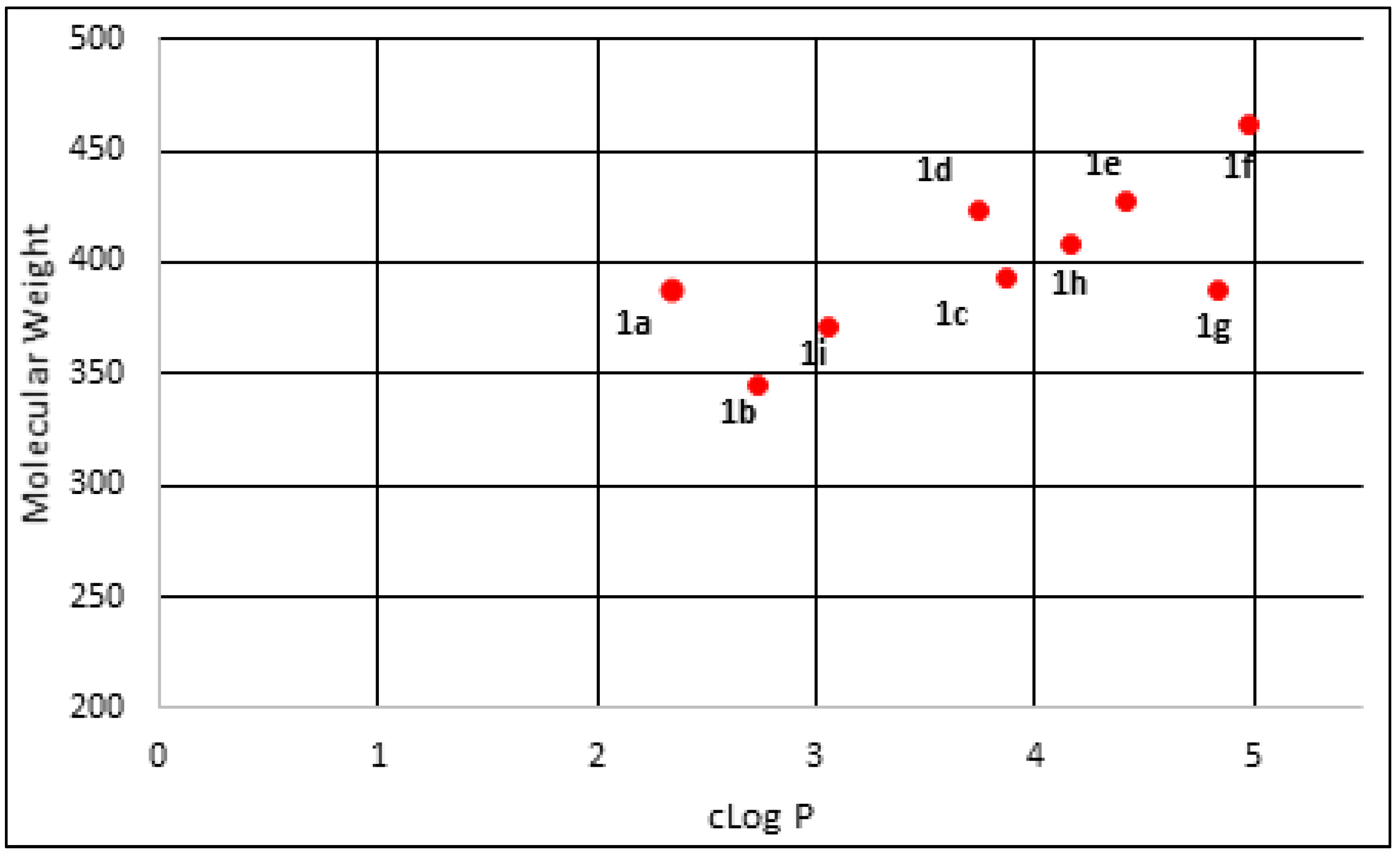
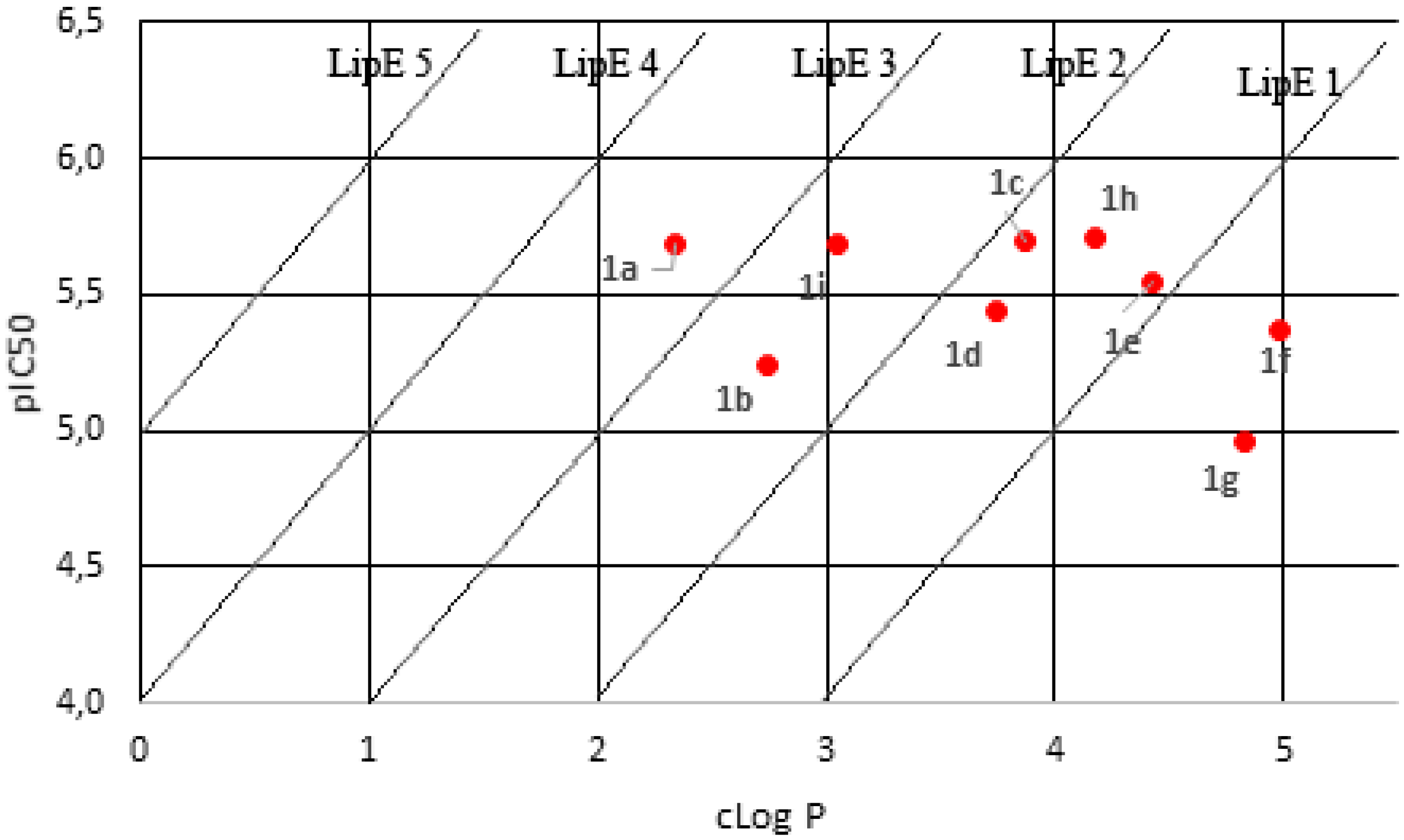
2.2. Physicochemical Properties Analysis
| Compound | Molecular Weight | pIC50 | cLog P | LipE a |
|---|---|---|---|---|
| 1a | 387.45 | 5.685 | 2.34 | 3.345 |
| 1b | 345.41 | 5.237 | 2.74 | 2.497 |
| 1c | 393.46 | 5.692 | 3.87 | 1.822 |
| 1d | 423.48 | 5.439 | 3.74 | 1.699 |
| 1e | 427.90 | 5.549 | 4.42 | 1.129 |
| 1f | 462.35 | 5.364 | 4.98 | 0.384 |
| 1g | 387.49 | 4.959 | 4.83 | 0.129 |
| 1h | 407.48 | 5.707 | 4.17 | 1.537 |
| 1i | 371.45 | 5.683 | 3.05 | 2.633 |
| CQ b | 515.86 | 6.343 | 3.73 | 2.613 |
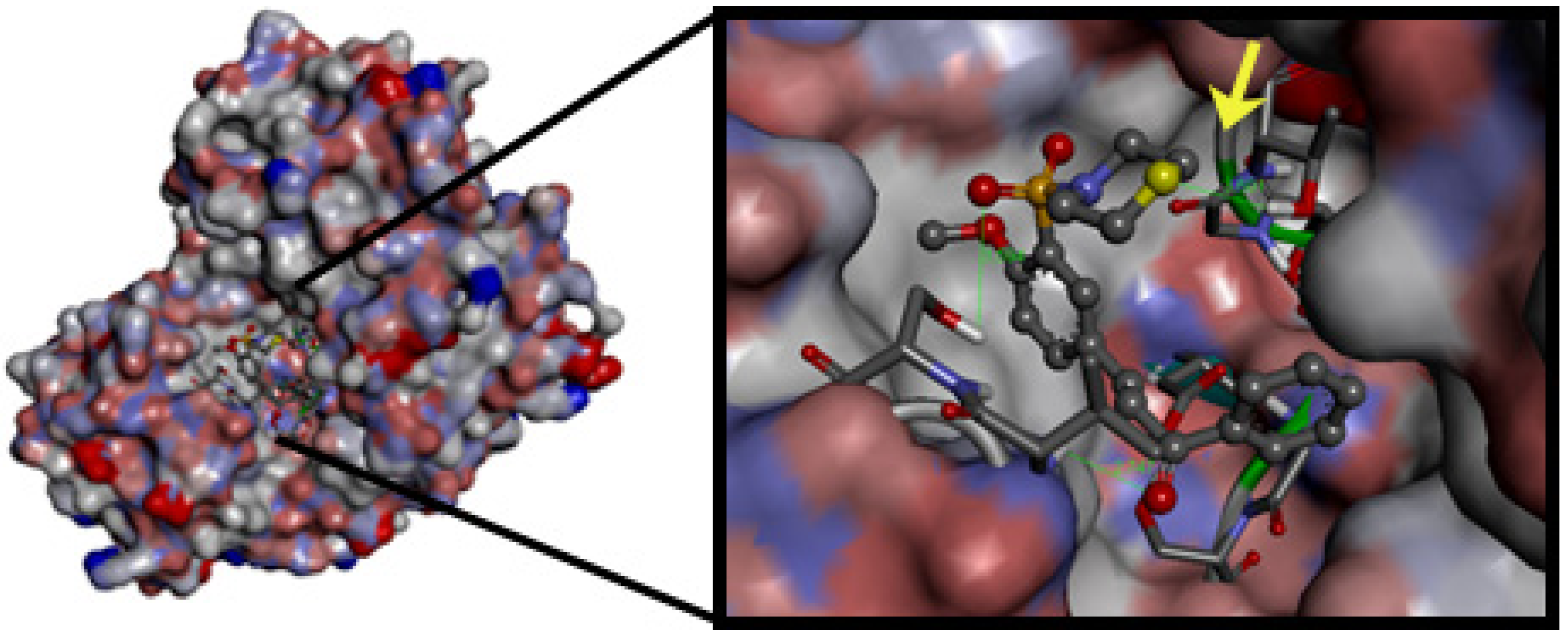
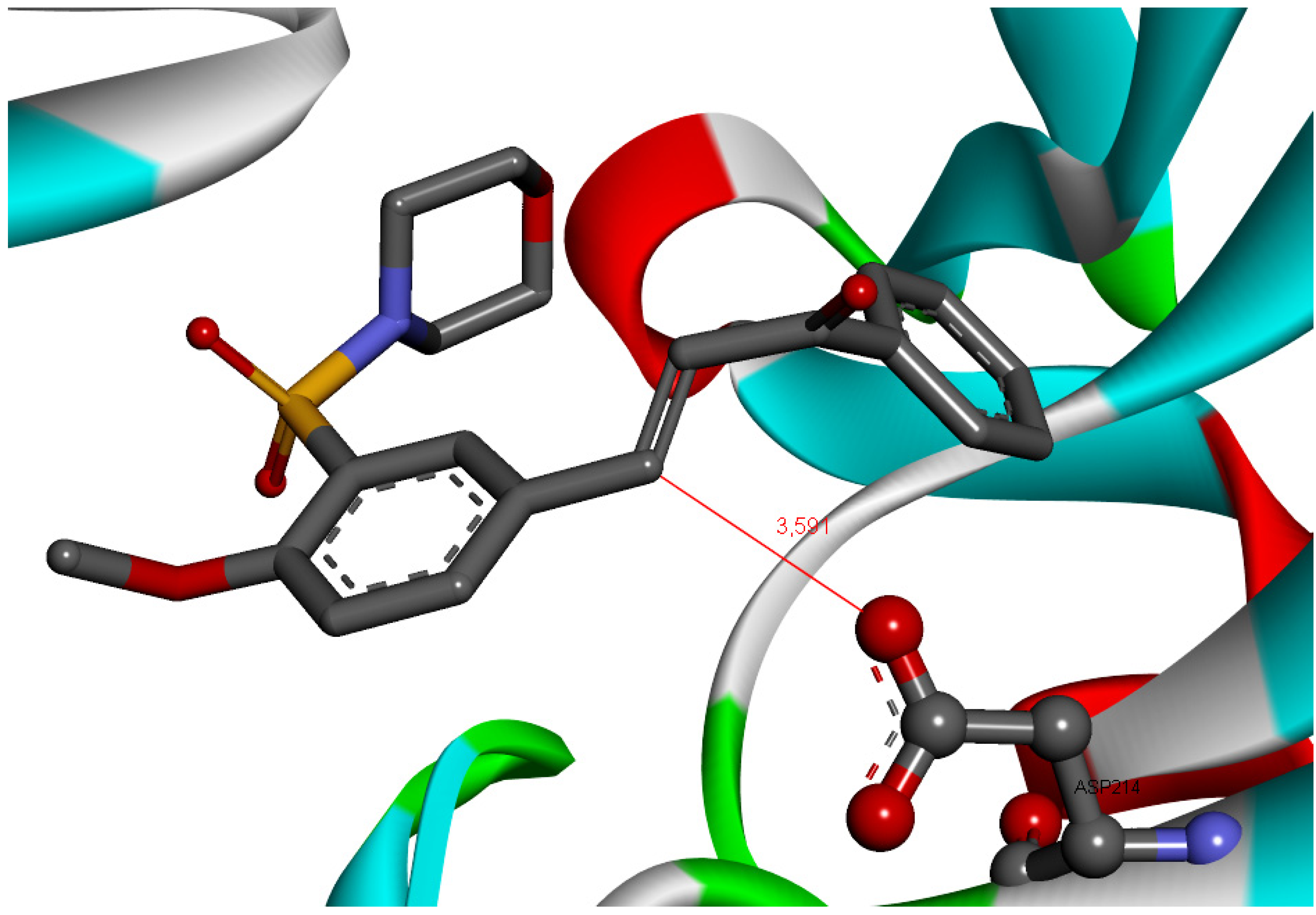
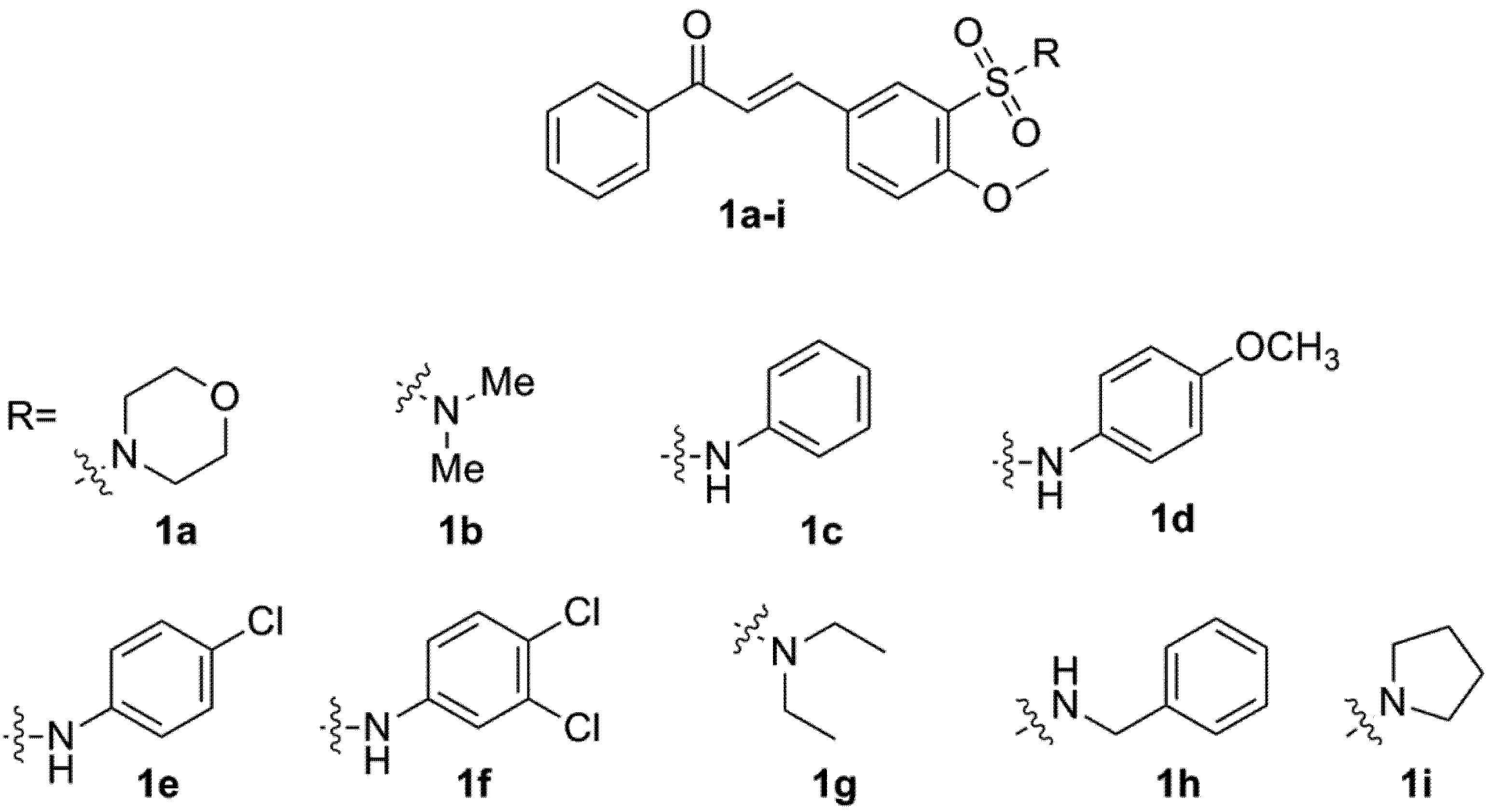
2.3. Docking of 4-Metoxychalcone Derivatives to Falcipain-2, Plasmepsin-2, and Plasmepsin-4
| Compound | ΔG Energy (Kcal.mol−1) | ||
|---|---|---|---|
| Falcipain-2 | Plasmepsin-2 | Plasmepsin-4 | |
| 1a | −4.9 | −7.3 | −6.9 |
| 1b | −5.5 | −7.2 | −7.0 |
| 1c | −6.2 | −8.0 | −7.4 |
| 1d | −6.1 | −7.8 | −7.3 |
| 1e | −6.1 | −8.0 | −7.5 |
| 1f | −6.5 | −8.5 | −7.6 |
| 1g | −5.5 | −6.6 | −6.2 |
| 1h | −5.0 | −7.7 | −6.9 |
| 1i | −5.5 | −7.1 | −7.1 |
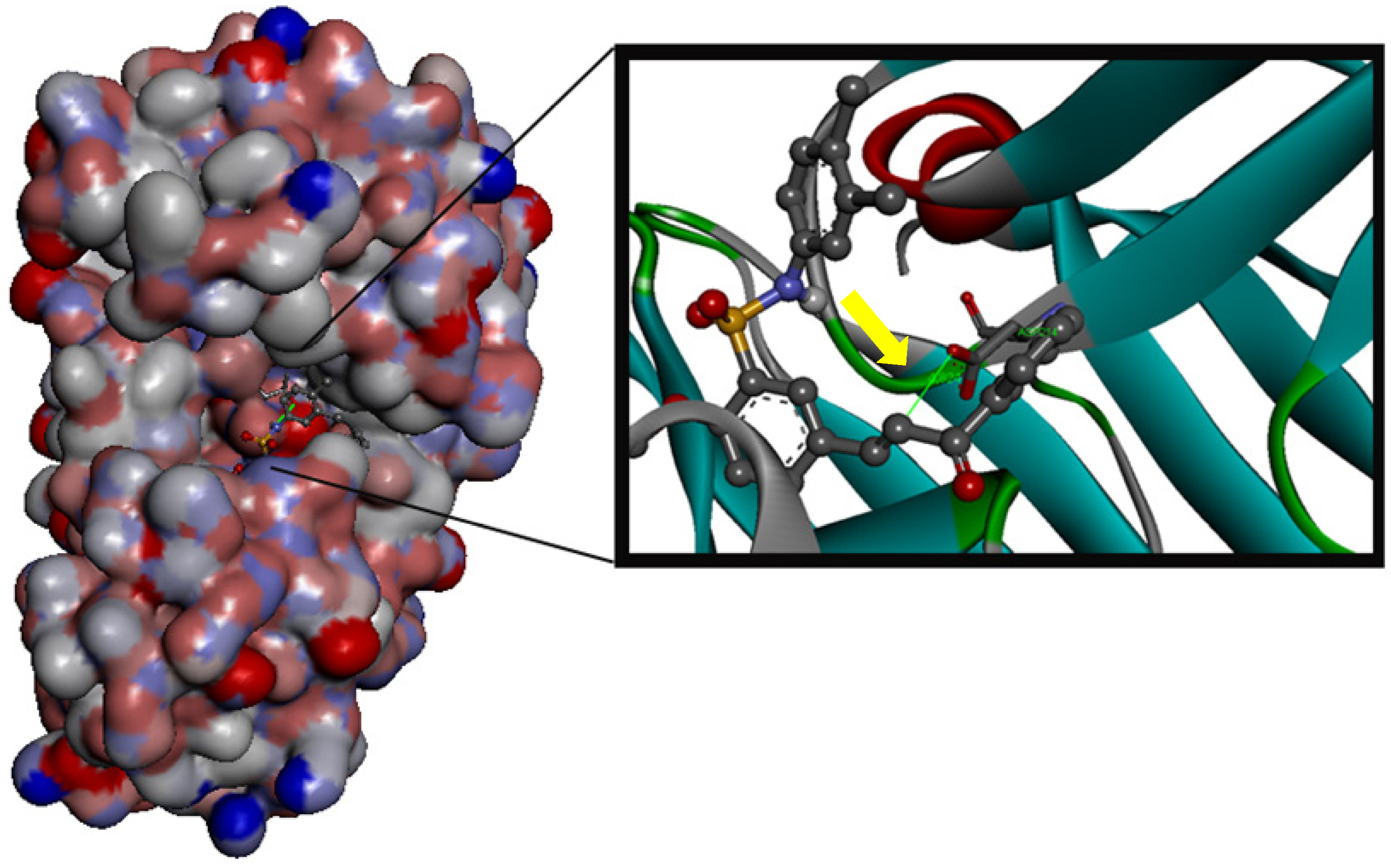
3. Experimental
3.1. Drug Samples
3.2. Biological Assays
3.2.1. Plasmodium Falciparum Continuous Culture
3.2.2. In vitro Antiplasmodial Activity
3.2.3. Cytotoxicity Assay
3.3. General Procedure of Docking
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- World Health Organization. World Malaria Report. 2011. Available online: http://www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html (accessed on 10 April 2013).
- Phyo, A.P.; Nkhoma, S.; Stepniewska, K.; Ashley, E.A.; Nair, S.; McGready, R.; ler Moo, C.; Al-Saai, S.; Dondorp, A.M.; Lwin, K.M.; et al. Emergence of artemisinin-resistant malaria on the western border of thailand: A longitudinal study. Lancet 2012, 379, 1960–1966. [Google Scholar] [CrossRef]
- Carrara, V.I.; Lwin, K.M.; Phyo, A.P.; Ashley, E.; Wiladphaingern, J.; Sriprawat, K.; Rijken, M.; Boel, M.; McGready, R.; Proux, S.; et al. Malaria burden and artemisinin resistance in the mobile and migrant population on the thai-myanmar border, 1999–2011: An observational study. PLoS Med. 2013, 10, e1001398. [Google Scholar] [CrossRef]
- Hartwig, C.L.; Lauterwasser, E.M.; Mahajan, S.S.; Hoke, J.M.; Cooper, R.A.; Renslo, A.R. Investigating the antimalarial action of 1,2,4-trioxolanes with fluorescent chemical probes. J. Med. Chem. 2011, 54, 8207–8213. [Google Scholar]
- Miller, L.H.; Ackerman, H.C.; Su, X.Z.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef]
- Da Silva, G.D.; da Silva, M.G.; Souza, E.M.; Barison, A.; Simões, S.C.; Varotti, F.P.; Barbosa, L.A.; Viana, G.H.; Villar, J.A. Design and synthesis of new chacones substituted with azide/triazole groups and analysis of their cytotoxicity towards hela cells. Molecules 2012, 17, 10331–10343. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Avula, S.R.; Palnati, G.R.; Singh, S.V.; Srivastava, K.; Puri, S.K.; Saxena, J.K. Synthesis and in vitro evaluation of new chloroquine-chalcone hybrids against chloroquine-resistant strain of plasmodium falciparum. Bioorg. Med. Chem. Lett. 2012, 22, 5455–5459. [Google Scholar] [CrossRef]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial activity of newly synthesized chalcone derivatives in vitro. Chem. Biol. Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef]
- Tadigoppula, N.; Korthikunta, V.; Gupta, S.; Kancharla, P.; Khaliq, T.; Soni, A.; Srivastava, R.K.; Srivastava, K.; Puri, S.K.; Raju, K.S.; et al. Synthesis and insight into the structure-activity relationships of chalcones as antimalarial agents. J. Med. Chem. 2013, 56, 31–45. [Google Scholar] [CrossRef]
- Moura, P.A.; Dame, J.B.; Fidock, D.A. Role of plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob. Agents Chemother. 2009, 53, 4968–4978. [Google Scholar] [CrossRef]
- Agrawal, V.K.; Srivastava, R.; Khadikar, P.V. Qsar studies on some antimalarial sulfonamides. Bioorg. Med. Chem. 2001, 9, 3287–3293. [Google Scholar] [CrossRef]
- Sharma, N.; Mohanakrishnan, D.; Shard, A.; Sharma, A.; Saima; Sinha, A.K.; Sahal, D. tilbene-chalcone hybrids: Design, synthesis, and evaluation as a new class of antimalarial scaffolds that trigger cell death through stage specific apoptosis. J. Med. Chem. 2012, 55, 297–311. [Google Scholar] [CrossRef]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Bhampidipati, R.; Kopinathan, A.; Smith, P.J.; Chibale, K. Enone- and chalcone-chloroquinoline hybrid analogues: In silico guided design, synthesis, antiplasmodial activity, in vitro metabolism, and mechanistic studies. J. Med. Chem. 2011, 54, 3637–3649. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Method. 2000, 44, 235–249. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Andrighetti-Fröhner, C.R.; de Oliveira, K.N.; Gaspar-Silva, D.; Pacheco, L.K.; Joussef, A.C.; Steindel, M.; Simões, C.M.; de Souza, A.M.; Magalhaes, U.O.; Afonso, I.F.; et al. Synthesis, biological evaluation and sar of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity. Eur J. Med. Chem. 2009, 44, 755–763. [Google Scholar] [CrossRef]
- Abad-Zapatero, C.; Metz, J.T. Ligand efficiency indices as guideposts for drug discovery. Drug Discov. Today 2005, 10, 464–469. [Google Scholar] [CrossRef]
- Ryckmans, T.; Edwards, M.P.; Horne, V.A.; Correia, A.M.; Owen, D.R.; Thompson, L.R.; Tran, I.; Tutt, M.F.; Young, T. Rapid assessment of a novel series of selective cb(2) agonists using parallel synthesis protocols: A lipophilic efficiency (lipe) analysis. Bioorg. Med. Chem. Lett. 2009, 19, 4406–4409. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving drug candidates by design: A focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Aguiar, A.C.; Santos, R.E.M.; Figueiredo, F.J.; Cortopassi, W.A.; Pimentel, A.S.; França, T.C.; Meneghetti, M.R.; Krettli, A.U. Antimalarial activity and mechanisms of action of two novel 4-aminoquinolines against chloroquine-resistant parasites. PLoS One 2012, 7, e37259. [Google Scholar] [CrossRef]
- Penna-Coutinho, J.; Cortopassi, W.A.; Oliveira, A.A.; França, T.C.; Krettli, A.U. Antimalarial activity of potential inhibitors of plasmodium falciparum lactate dehydrogenase enzyme selected by docking studies. PLoS One 2011, 6, e21237. [Google Scholar]
- Hann, M.M.; Keserü, G.M. Finding the sweet spot: The role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a set of simple, interpretable admet rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Tarcsay, A.; Nyíri, K.; Keseru, G.M. Impact of lipophilic efficiency on compound quality. J. Med. Chem. 2012, 55, 1252–1260. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Taranto, A.G.; Carvalho, P.; Avery, M.A. Qm/qm studies for michael reaction in coronavirus main protease (3cl pro). J. Mol. Graph. Model. 2008, 27, 275–285. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. 1976. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
- Batista, R.; García, P.A.; Castro, M.A.; Miguel Del Corral, J.M.; Speziali, N.L.; de P Varotti, F.; de Paula, R.C.; García-Fernández, L.F.; Francesch, A.; San Feliciano, A.; et al. Synthesis, cytotoxicity and antiplasmodial activity of novel ent-kaurane derivatives. Eur. J. Med. Chem. 2013, 62, 168–176. [Google Scholar] [CrossRef]
- Fundação Hemominas-Hemonúcleo Divinópolis. Available online: http://www.hemominas.mg.gov.br (accessed on 1 August 2013).
- Lambros, C.; Vanderberg, J.P. Synchronization of plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Carvalho, L.H.; Brandão, M.G.; Santos-Filho, D.; Lopes, J.L.; Krettli, A.U. Antimalarial activity of crude extracts from brazilian plants studied in vivo in plasmodium berghei-infected mice and in vitro against plasmodium falciparum in culture. Braz. J. Med. Biol. Res. 1991, 24, 1113–1123. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Park, J.G.; Kramer, B.S.; Steinberg, S.M.; Carmichael, J.; Collins, J.M.; Minna, J.D.; Gazdar, A.F. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987, 47, 5875–5879. [Google Scholar]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Stewart, J.J. Optimization of parameters for semiempirical methods v: Modification of nddo approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Oliveira, M.E.; Cenzi, G.; Nunes, R.R.; Andrighetti, C.R.; De Sousa Valadão, D.M.; Dos Reis, C.; Simões, C.M.O.; Nunes, R.J.; Júnior, M.C.; Taranto, A.G.; et al. Antimalarial Activity of 4-Metoxychalcones: Docking Studies as Falcipain/Plasmepsin Inhibitors, ADMET and Lipophilic Efficiency Analysis to Identify a Putative Oral Lead Candidate. Molecules 2013, 18, 15276-15287. https://doi.org/10.3390/molecules181215276
De Oliveira ME, Cenzi G, Nunes RR, Andrighetti CR, De Sousa Valadão DM, Dos Reis C, Simões CMO, Nunes RJ, Júnior MC, Taranto AG, et al. Antimalarial Activity of 4-Metoxychalcones: Docking Studies as Falcipain/Plasmepsin Inhibitors, ADMET and Lipophilic Efficiency Analysis to Identify a Putative Oral Lead Candidate. Molecules. 2013; 18(12):15276-15287. https://doi.org/10.3390/molecules181215276
Chicago/Turabian StyleDe Oliveira, Michael Eder, Gisele Cenzi, Renata Rachide Nunes, Carla Regina Andrighetti, Denia Mendes De Sousa Valadão, Cláudia Dos Reis, Cláudia Maria Oliveira Simões, Ricardo José Nunes, Moacyr Comar Júnior, Alex Gutterres Taranto, and et al. 2013. "Antimalarial Activity of 4-Metoxychalcones: Docking Studies as Falcipain/Plasmepsin Inhibitors, ADMET and Lipophilic Efficiency Analysis to Identify a Putative Oral Lead Candidate" Molecules 18, no. 12: 15276-15287. https://doi.org/10.3390/molecules181215276




