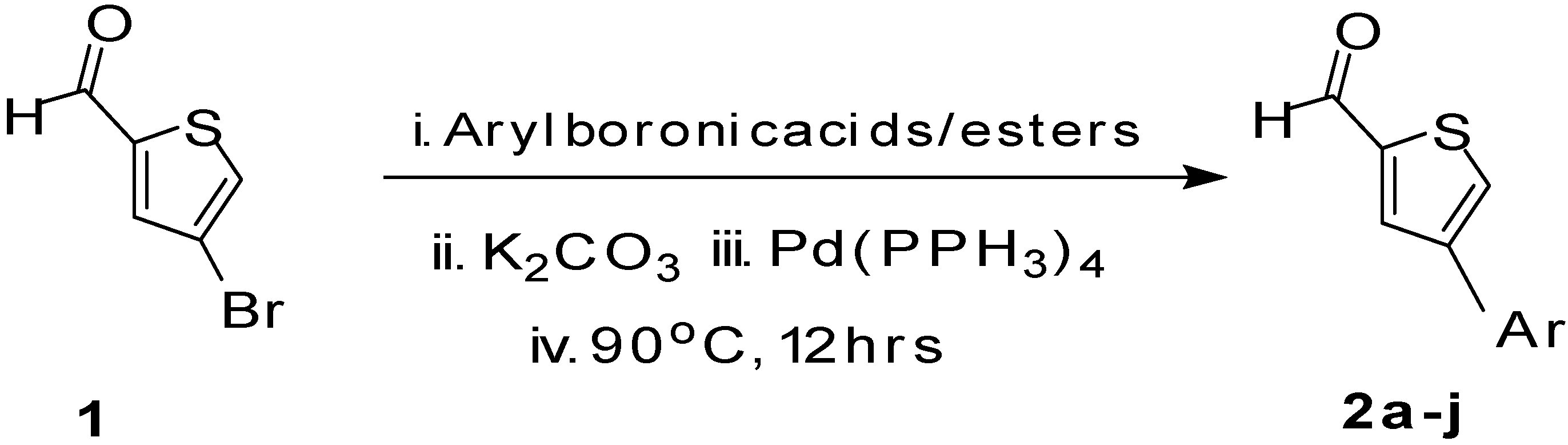
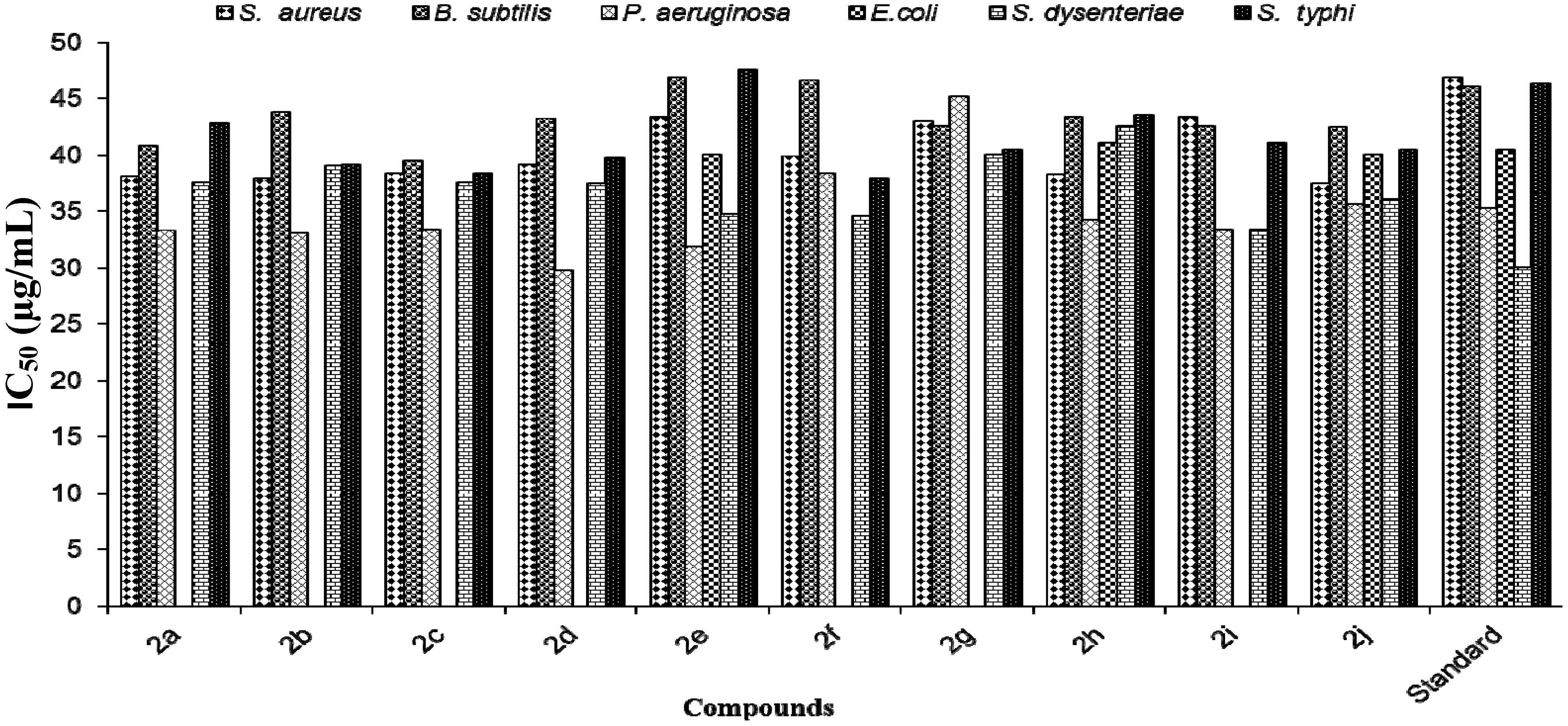
3.3. Characterization Data
4-Phenylthiophene-2-carbaldehyde (2a). Mp: 57–59 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.95 (s, 1H), 8.01 (s, 1H-thiophene), 7.83 (s, 1H-thiophene), 7.5 (d, J = 1.5 Hz, 1H-aryl), 7.44–7.39 (m, 2H-aryl), 7.36–7.31 (m, 2H-aryl). 13C-NMR (CDCl3 + CD3OD): δ = 126.30, 127.19, 128.09, 128.77, 129.06, 129.63, 130.86, 134.34, 134.73, 143.64, 144.37, 183.01; EIMS (m/z +ion mode): 188 [M‒H+]; [M‒CHO]+ = 160.01; [M‒C7H6]+ = 102.1; [M‒phenyl]+ = 115.1. Anal. Calcd. for C11H8OS (188.03): C, 70.18; H, 4.28; O, 8.50; S, 17.03. Found: C, 70.22; H, 4.26; O, 8.55; S, 17.12%.
4-(3,5-Bis(trifloromethyl)phenyl)thiophene-2-carbaldehyde (2b). Mp: 95–97 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.99 (s, 1H), 8.06 (s, 1H-aryl), 7.99 (s, 2H-aryl), 7.84 (s, 1H-thiophene), 7.24 (s, 1H-thiophene). 13C-NMR (CDCl3 + CD3OD): δ = 121.57, 126.32, 131.31, 132.37, 132.81, 133.76, 136.42, 140.44, 145.39, 182.56; EIMS (m/z +ion mode): 323 [M‒H+]; [M‒2C]+ = 226.0; [M‒O]+ = 207; [M‒F2,C7H6O]+ = 182.1; [M‒Ph‒3-CF3‒5-CF3]+ = 113. Anal. Calcd. for C13H6F6OS (324.00): C, 48.16; H, 1.87; F, 35.16; O, 4.93; S, 9.89. Found: C, 48.22; H, 1.93; F, 35.05; O, 4.81; S, 9.78%.
5-Chloro-2,2-bisthiophene-5-carbaldehyde (2c). Mp: 229–231 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.84 (s, 1H), 8.00 (d, J = 1.1 Hz, 1H-thiophene), 7.83 (d, J = 1.2 Hz, 1H-thiophene), 7.51 (d, J = 6.9 Hz, 1H-thiophene), 6.7 (d, J = 3.9 Hz, 1H-thiophene). 13C-NMR (CDCl3 + CD3OD): δ = 123.4, 125.2, 126.3, 127.5, 136.7, 138.1, 140.2, 143.4, 182.4; EIMS (m/z +ion mode): 229 [M‒H+]; [M‒CHO]+ = 201.00; [M‒O]+ = 211.00; [M‒thiophene]+ = 147.17. Anal. Calcd. for C9H5OS2 (192.98): C, 55.92; H, 2.61; O, 8.28; S, 33.18. Found: C, 55.97; H, 2.65; O, 8.32; S, 33.13%.
3-(5-Formylthiophene-3-yl)-5-(trifloromethyl)benzonitrile (2d). Mp: 252–254 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.97 (s, 1H), 8.08 (s, 1H-aAryl), 8.03 (s, 1H-aryl), 7.61–7.53 (m, 2H-thiophene, 1H-aryl). 13C-NMR (CDCl3 + CD3OD): δ = 22.69, 116.97, 127.09, 129.65, 131.54, 132.79, 133.40, 134.69, 137.95, 143.65, 144.30, 182.98; EIMS (m/z +ion mode): 280.58 [M‒H+]; [M‒CN]+ = 256; [M‒F2]+ = 243.91; [M‒thiophene]+ = 198.70. Anal. Calcd. for C13H6F3NOS (281.05): C, 55.52; H, 2.15; F, 20.26; N, 4.98; O, 5.69; S, 11.40. Found: C, 55.57; H, 2.19; F, 20.21; O, 5.64; S, 11.48%.
4-p-Tolylthiophene-2-carbaldehyde (2e). Mp: 58–60 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.94 (s, 1H), 7.99 (s, 1H-thiophene), 7.791 (s, 1H-thiophene), 7.4 (d, J = 7.5 Hz, 1H-aryl), 7.2 (d, J = 7.8 Hz, 1H aryl), 2.37 (s, 3H). 13C-NMR (CDCl3 + CD3OD): δ = 22.67, 126.18, 129.06, 129.74, 131.58, 134.69, 137.95, 143.65, 144.30, 182.98; EIMS (m/z +ion mode): 202.01 [M‒H+]; [M‒CH3]+ = 187.05; [M‒CHO]+ = 173.98; [M‒Ph]+ = 111.76; [M‒thiophenecarbaldehyde]+ = 77.58. Anal. Calcd. for C12H10OS (202.01): C, 17.25; H, 4.98; O, 7.91; S, 15.85. Found: C, 17.28; H, 4.92; O, 7.93; S, 15.82%.
4-(4-Methoxyphenyl)thiophene-2-carbaldehyde (2f). Mp: 68–70 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.84 (s, 1H), 8.16 (s, 1H-thiophene), 8.06 (s, 1H-thiophene), 7.68 (m, 2H-aryl), 7.05 (d, J = 8.4 Hz, 2H-aryl), 3.83 (s, 3H-OCH3). 13C-NMR (CDCl3 + CD3OD): δ = 55.38, 114.48, 127.72, 128.63, 132.18, 134.58, 143.38, 144.28, 159.57, 183.01; EIMS (m/z +ion mode): 217.86 [M‒H+]; [M‒CHO]+ = 190.56; [M‒OCH3]+ = 188.03; [M‒Ph]+ = 141.76; [M‒thiophenecarbaldehyde]+ = 108.07. Anal. Calcd. for C12H10O2S (218.02): C, 66.03; H, 4.62; O, 14.66; S, 14.69. Found: C, 66.12; H, 4.66; O, 14.59; S, 14.72%.
4-(3,5-Dimethylphenyl)thiophene-2-carbaldehyde (2g). Mp: 71–73 °C; 1H-NMR (CDCl3 +CD3OD): δ = 9.94 (s, 1H), 8.00 (s, 1H-thiophene), 7.79 (s, 1H-thiophene), 7.18 (s, 2H-aryl), 6.98 (s, 1H-aryl), 2.35 (s, 6H, 2CH3). 13C-NMR (CDCl3 + CD3OD): δ = 21.35, 124.24, 129.47, 129.70, 134.28, 134.98, 138.66, 143.91, 144.23, 183.01; EIMS (m/z +ion mode): 217 [M‒H+]; [M‒CHO]+ = 189.00; [M‒2CH3]+ = 189.00. Anal. Calcd. for C13H12OS (216.06): C, 72.19; H, 5.59; O, 7.40; S, 14.82. Found: C, 72.24; H, 5.62; O, 7.38; S, 14.85%.
4-(3,4-Dichlorophenyl)thiophene-2-carbaldehyde (2h). Mp: 158–160 °C; 1H-NMR (CDCl3 + CD3OD):δ = 9.95 (s, 1H), 7.94 (s, 1H-thiophene), 7.79 (s, 1H-thiophene), 7.62 (s, 1H-Aryl), 7.59 (d, J = 2.0 Hz, 1H-Aryl), 7.42 (d, J = 2.4 Hz, 1H-Aryl). 13C-NMR (CDCl3 + CD3OD): δ = 117.32, 121.87, 126.10, 129.91, 131.74, 134.15, 141.31, 144.81, 156.63, 159.12, 182.72; EIMS (m/z +ion mode): 256 [M‒H+]; [M‒CO]+ = 229.17.00; [M‒thiophene]+ = 85.00. Anal. Calcd. for C11H6Cl2OS (255.96): C, 51.38; H, 2.35; Cl, 27.58; O, 6.22; S, 12.47. Found: C, 51.40; H, 2.38; Cl, 27.63; O, 6.18; S, 12.51%.
4-(3-Chloro-4-fluorophenyl)thiophene-2-carbaldehyde (2i). Mp: 108–110 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.95 (s, 1H), 7.83 (s, 1H-thiophene), 7.50 (s, 1H-thiophene), 7.48 (s, 1H-aryl), 7.41 (d, J = 2.0 Hz, 1H-Aryl), 7.39 (d, J = 2.0 Hz, 1H-aryl). 13C-NMR (CDCl3 + CD3OD): δ = 117.20, 126.07, 128.53, 129.90, 131.73, 134.13, 141.31, 144.81, 156.63, 159.12, 182.71; EIMS (m/z +ion mode): 240.25 [M‒H+]; [M‒CHO]+ = 213.08; [M‒F]+ = 195.08. Anal. Calcd. for C11H6ClFOS (239.98): C, 54.89; H, 2.51; Cl, 14.73; F, 7.89 O, 6.65; S, 13.32. Found: C, 54.93; H, 2.51; Cl, 14.69; F, 7.91; O, 6.59; S, 13.36S%.
5-Methyl-2,3-bisthiophene-5-carbaldehyde (2j). Mp: 218–220 °C; 1H-NMR (CDCl3 + CD3OD): δ = 9.92 (s, 1H), 7.87 (s, 1H-thiophene), 7.65 (s, 1H-thiophene), 7.01 (d, J = 3.2 Hz, 1H-thiophene), 4.10 (d, J = 6.8 Hz, 1H-thiophene), 2.02 (s, 3H-methyl). 13C-NMR (CDCl3 + CD3OD): δ = 15.34, 21.02, 22.67, 29.68, 60.38, 123.92, 126.05, 127.70, 133.91, 182.81; EIMS (m/z +ion mode): 189.67 [M‒H+]; [M‒CH3]+ = 175.87; [M‒CO]+ = 161.67; [M-thiophene]+ = 107.75. Anal. Calcd. for C10H8OS2 (208.02): C, 57.66; H, 3.37; O, 7.68; S, 30.79. Found: C, 57.61; H, 3.42; O, 7.64; S, 30.82%.