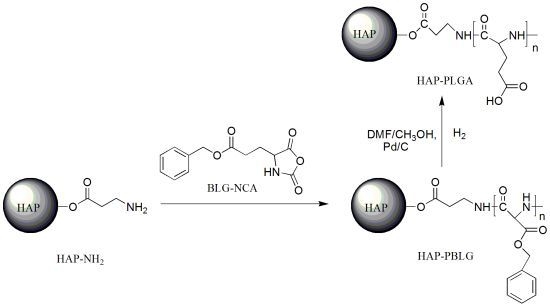
The Synthesis and Characterization of Hydroxyapatite-β-Alanine Modified by Grafting Polymerization of γ-Benzyl-L-glutamate-N-carboxyanhydride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Grafting Polymerization of BLG-NCA on HAP-Ala Crystals
| Sample ID | Yield (%) | Mn a | PDI a | Percentage grafting (%) b | Ca content (%) c | |
|---|---|---|---|---|---|---|
| Theoretical value | Measured value | |||||
| HAP-NH2 | -- | -- | -- | -- | 38.46 | 15.89 |
| HAP-5 | 54.4 | -- | -- | 39.31 | 2.65 | 4.03 |
| HAP-10 | 60.6 | -- | -- | 66.36 | 1.45 | 2.56 |
| HAP-20 | 61.8 | -- | -- | 70.03 | 0.76 | 1.10 |
| HAP-50 | 85.0 | 148,000 | 1.01 | 76.79 | 0.31 | 0.44 |
| HAP-100 | 70.8 | 142,000 | 1.02 | 83.18 | 0.16 | 0.23 |
| HAP-200 | 81.8 | 131,000 | 1.02 | 80.37 | 0.08 | 0.23 |
| HAP-300 | 70.2 | 153,000 | 1.03 | 78.15 | 0.05 | 0.33 |
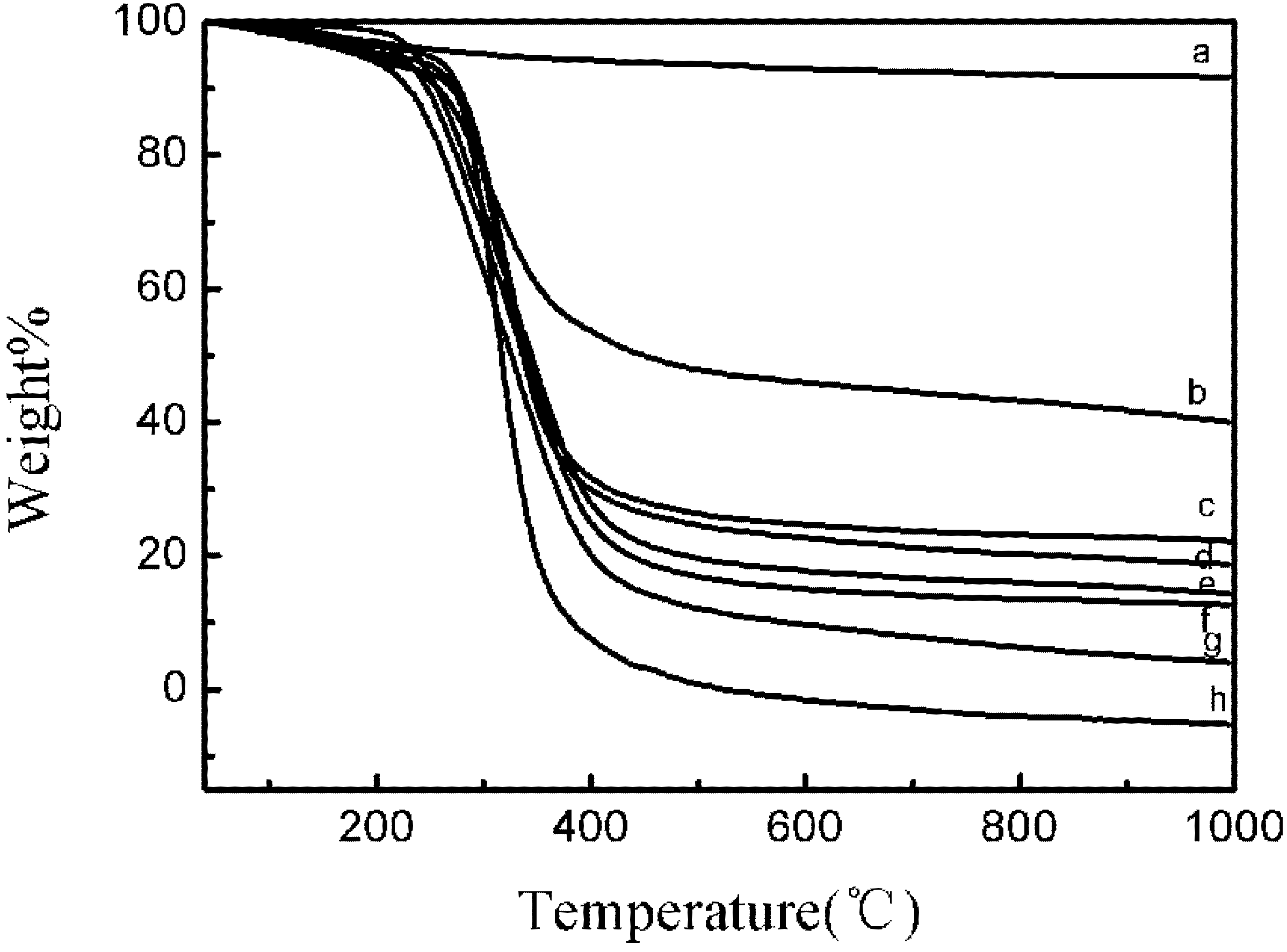
2.2. The Synthesis of Hydroxyapatite-β-alanine-poly (l-glutamic acid) (HAP-PLGA)
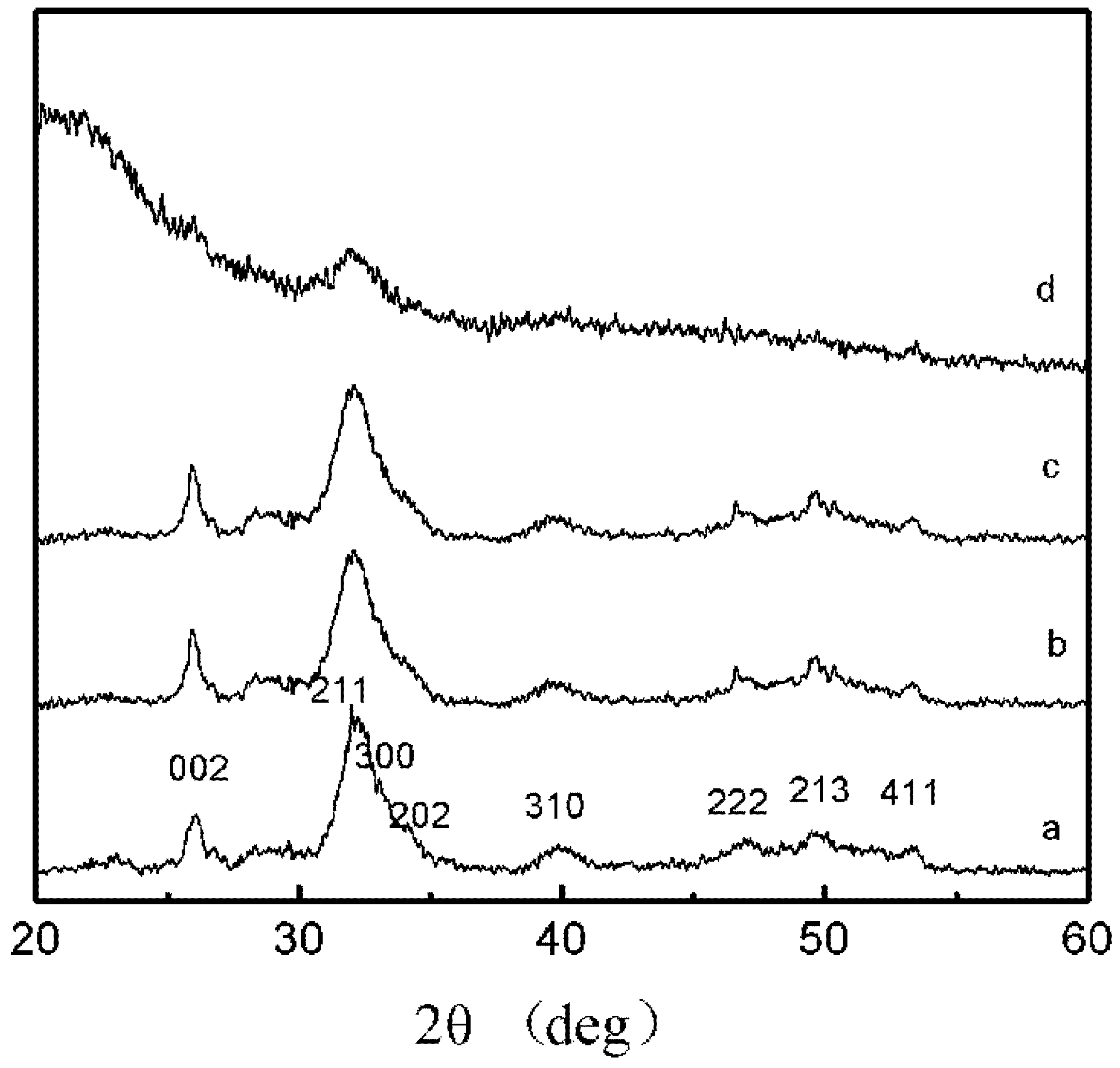
2.3. XRD Patterns
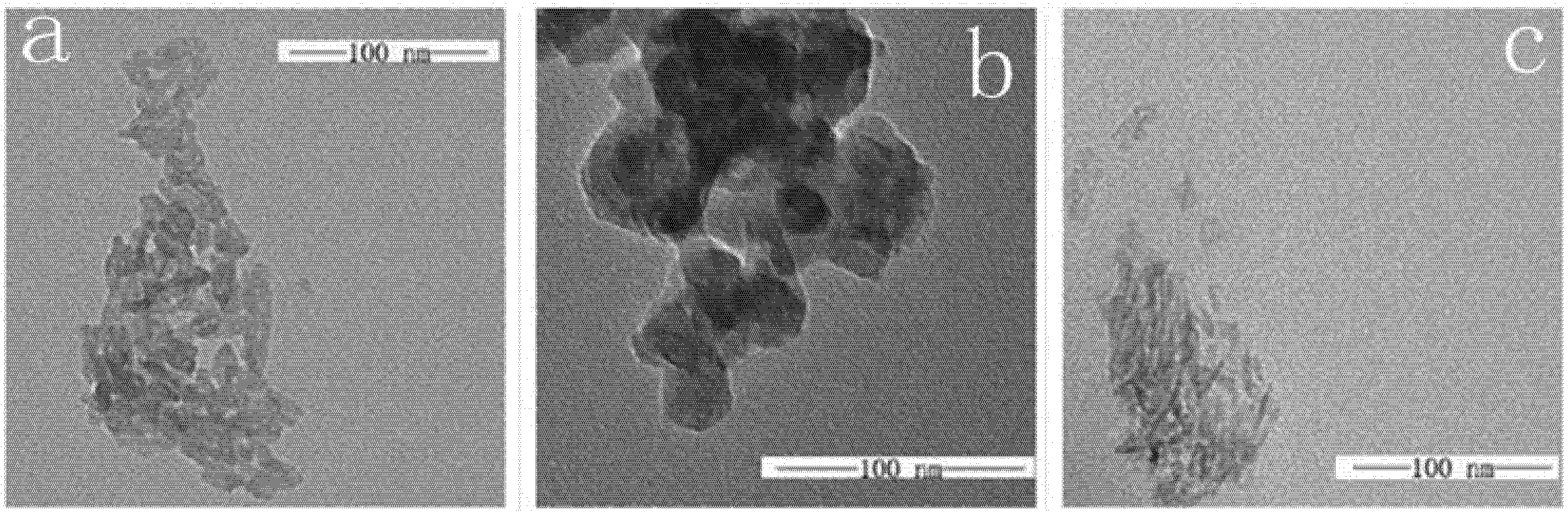
2.4. TEM
2.5. 1H-NMR Spectra
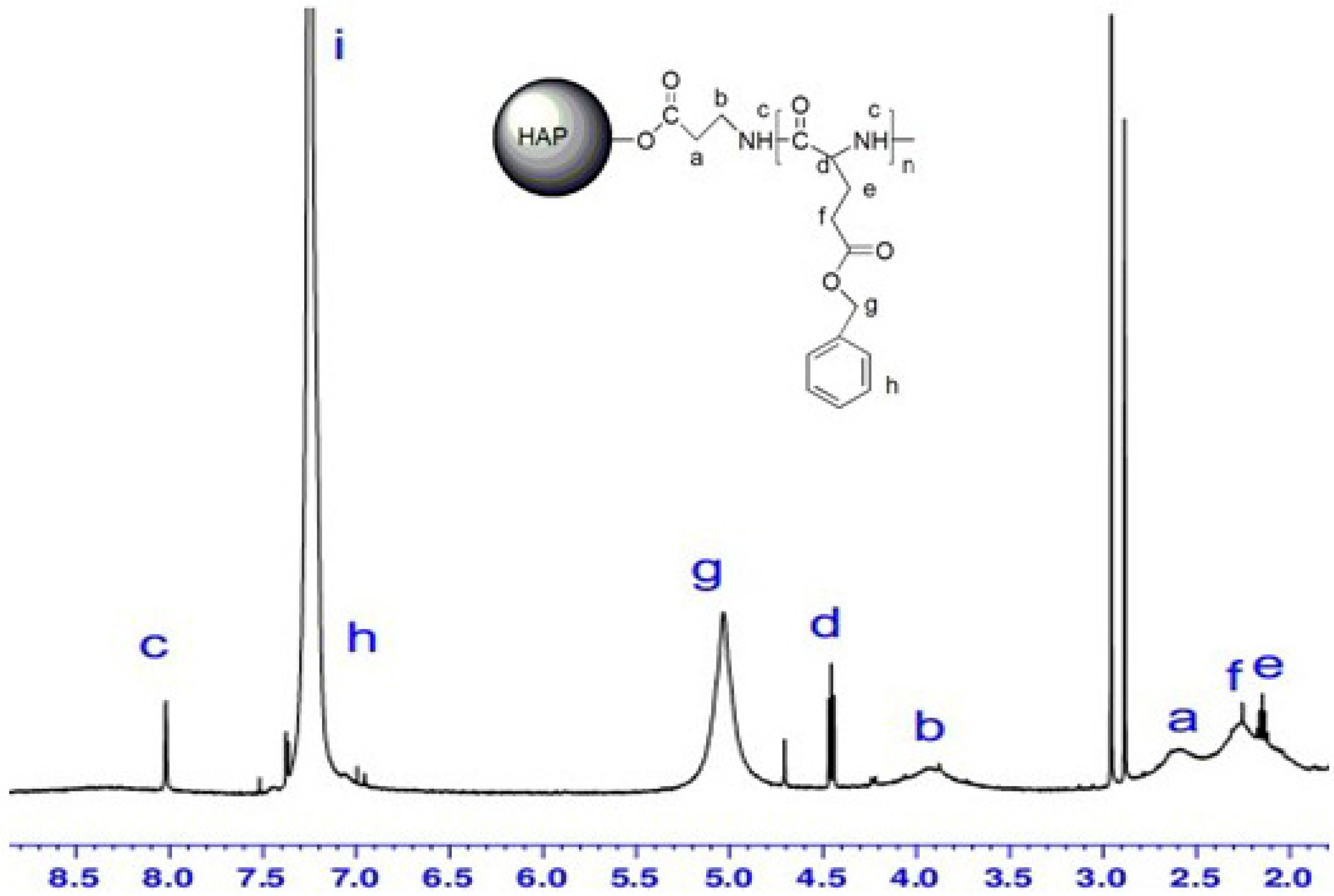
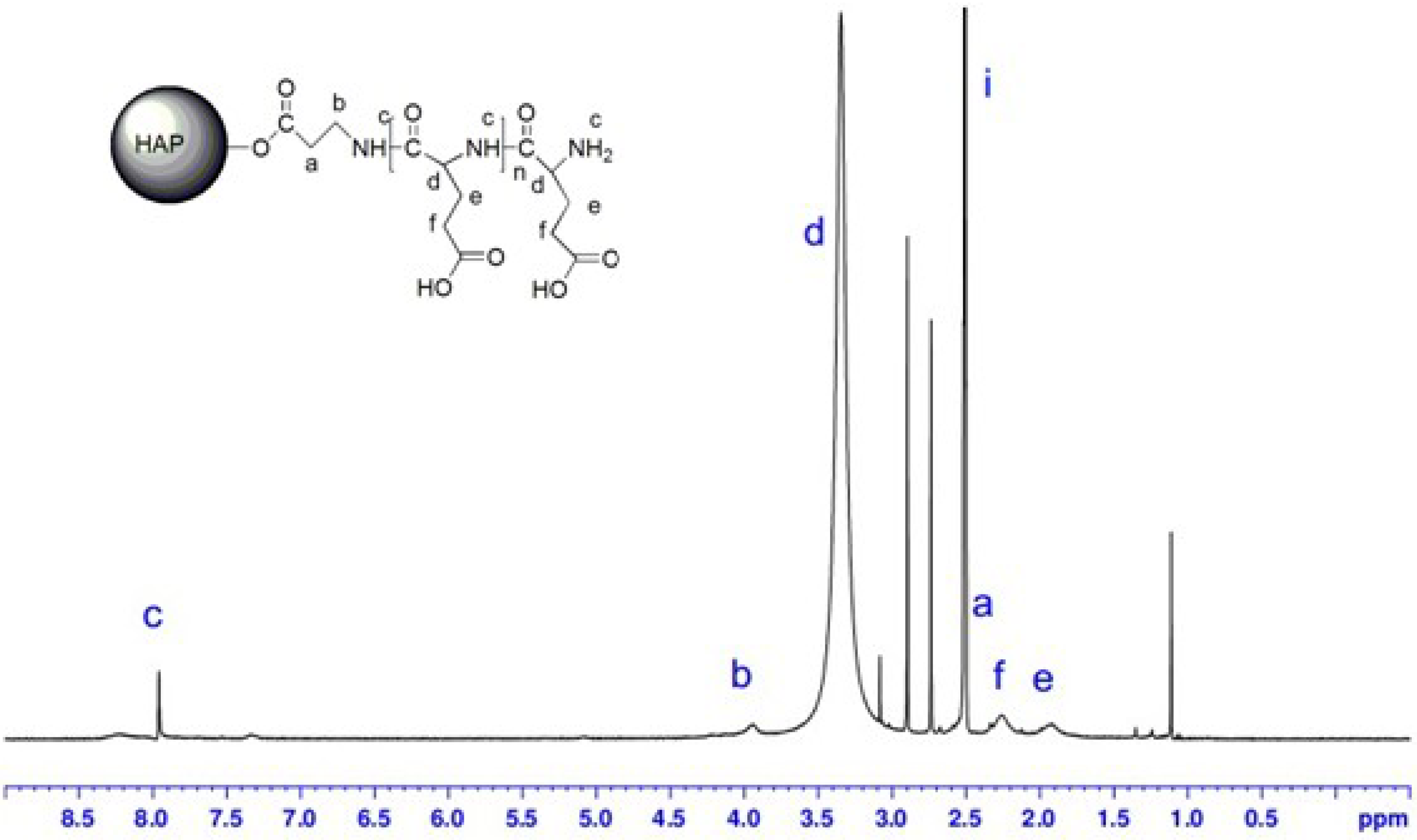
2.6. FTIR Spectra

3. Experimental
3.1. Materials
3.2. Synthesis of HAP-PBLG
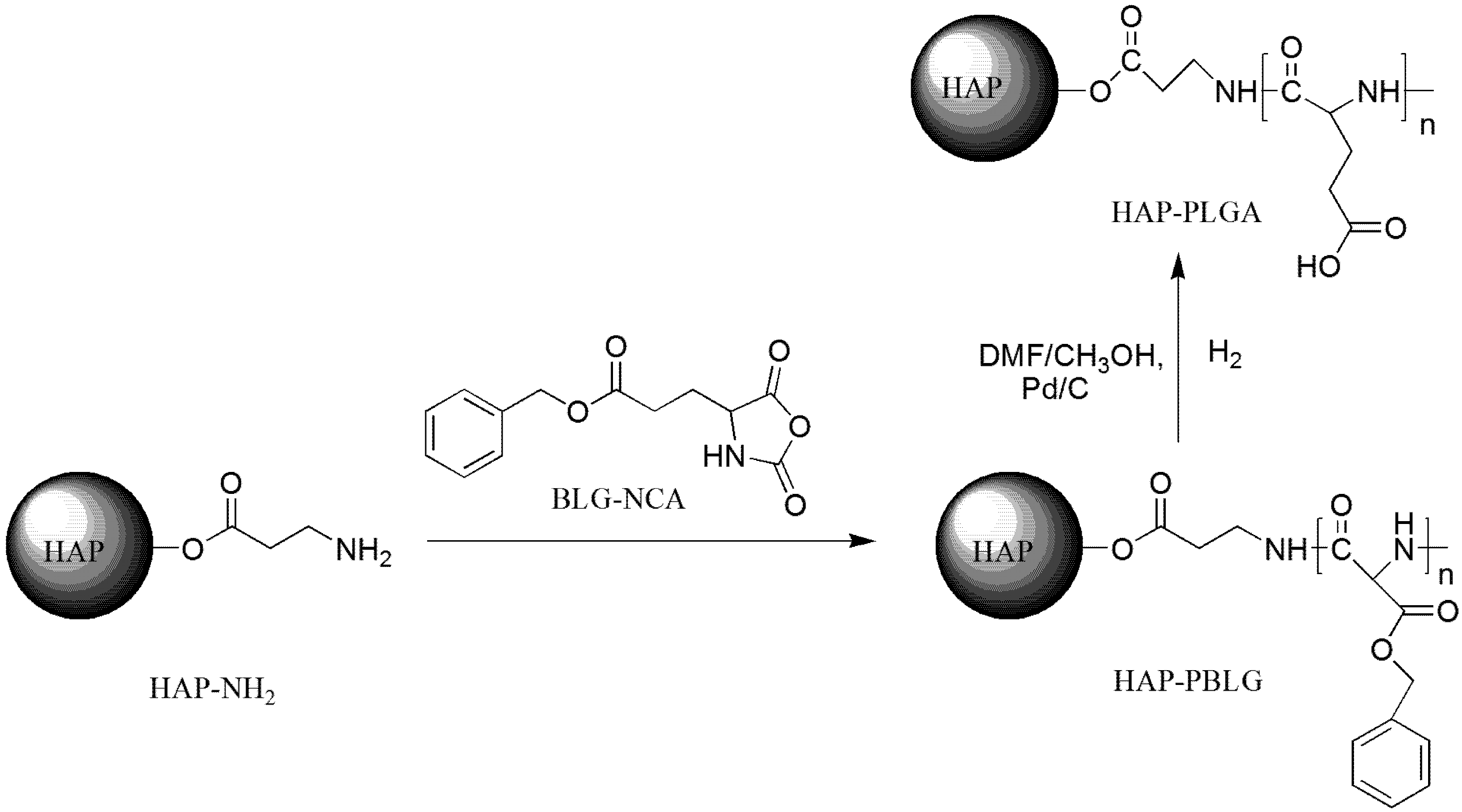
| Sample ID | BLG-NCA/HAP-NH2 |
|---|---|
| HAP-5 | 5/1 |
| HAP-10 | 10/1 |
| HAP-20 | 20/1 |
| HAP-50 | 50/1 |
| HAP-100 | 100/1 |
| HAP-200 | 200/1 |
| HAP-300 | 300/1 |
3.3. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bouyer, E.; Gitzhofer, F.; Boulos, M.I. Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 2000, 11, 523–531. [Google Scholar] [CrossRef]
- Pielichowsa, K.; Blazewicz, S. Bioactive polymer/hydroxyapatite (nano) composites for bone tissue regeneration. Adv. Polym. Sci. 2010, 232, 97–207. [Google Scholar]
- Wang, S.G.; Wen, S.H.; Shen, M.W.; Guo, R.; Cao, X.Y.; Wang, J.H.; Shi, X.Y. Aminopropyltriethoxysilane-mediated surface functionalization of hydroxyapatite nanoparticles: Synthesis, characterization, and in vitro toxicity assay. Nanomedicine 2011, 6, 3449–3459. [Google Scholar]
- Lee, S.C.; Choi, H.W.; Lee, H.J.; Kim, K.J.; Chang, J.H.; Kim, S.Y.; Choi, J.; Oh, K.; Jeong, Y.K. In-situ synthesis of reactive hydroxyapatite nano-crystals for a novel approach of surface grafting polymerization. J. Mater. Chem. 2007, 17, 174–180. [Google Scholar]
- Ethirajan, A.; Ziener, U.; Landfester, K. Surface-functionalized polymeric nanoparticles as templates for biomimetic mineralization of hydroxyapatite. Chem. Mater. 2009, 21, 2218–2225. [Google Scholar] [CrossRef]
- Roohani, S.R.; Nouri, S.N.; Lu, Z.F.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite–PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, H.W.; Kim, K.J.; Lee, S.C. Modification of hydroxyapatite nanosurfaces for enhanced colloidal stability and improved interfacial adhesion in nanocomposites. Chem. Mater. 2006, 18, 5111–5118. [Google Scholar] [CrossRef]
- Verma, D.; Katti, K.S.; Katti, D.R. Effect of biopolymers on structure of hydroxyapatite and interfacial interactions in biomimetically synthesized hydroxyapatite/biopolymer nanocomposites. Ann. Biomed. Eng. 2008, 36, 1024–1032. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Hay, I. Adsorption of molecules of biological interest onto hydroxyapatite. Calcif. Tissue Int. 1984, 36, 48–59. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.D.; Yang, S.; Yu, Q.F.; Wang, Z.L.; Zhang, Q.P. Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Mater. Lett. 2011, 65, 572–574. [Google Scholar]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Giardino, R. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials 2006, 27, 4428–4433. [Google Scholar] [CrossRef]
- Sakuragi, M.; Kitajima, T.; Nagmune, T.; Ito, Y. Recombinant hBMP4 incorporated with non-canonical amino acid for binding to hydroxyapatite. Biotechnol. Lett. 2011, 33, 1885–1890. [Google Scholar]
- Weiger, M.C.; Park, J.J.; Doy, M.D.; Stafford, C.M.; Karim, A.; Becker, M.L. Quantification of the binding affinity of a specific hydroxyapatite binding peptide. Biomaterials 2010, 31, 2955–2963. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, H.W.; Lee, H.J.; Chang, H.J.; Choi, J.; Kim, K.J.; Lim, H.J.; Junc, Y.J.; Lee, S.C. Designing a highly bioactive 3D bone-regenerative scaffold by surface immobilization of nano-hydroxyapatite. J. Mater. Chem. 2008, 18, 4994–5001. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Coupling of therapeutic molecules onto surface modified coralline hydroxyapatite. Biomaterials 2004, 25, 3073–3080. [Google Scholar] [CrossRef]
- Liu, Q.; de Wijn, J.R.; de Groot, K.; van Blitterswijk, C.A. Surface modification of nano-apatite by grafting organic polymer. Biomaterials 1998, 19, 1067–1072. [Google Scholar] [CrossRef]
- Yao, X.; Yao, H.W.; Li, G.Y.; Li, Y.T. Biomimetic synthesis of needle-like nano-hydroxyapatite templated by double-hydrophilic block copolymer. J. Mater. Sci. 2010, 45, 1930–1936. [Google Scholar] [CrossRef]
- Okabe, Y.; Kurihara, S.; Yajima, T.; Seki, Y.; Nakamura, I.; Takanob, I. Formation of super-hydrophilic surface of hydroxyapatite by ion implantation and plasma treatment. Surf. Coating. Tech. 2005, 196, 303–306. [Google Scholar] [CrossRef]
- Taguchi, T.; Muraoka, Y.; Matsuyama, H.; Kishida, A.; Akashi, M. Apatite coating on hydrophilic polymer-grafted poly(ethylene) films using an alternate soaking process. Biomaterials 2000, 22, 53–58. [Google Scholar] [CrossRef]
- Li, C.F.; Ge, X.L.; Liu, S.G.; Li, G.C.; Zhang, A.J.; Bai, J.H.; Su, C.H.; Ding, R. Redispersible dried hydroxyapatite particles with grafted pH-sensitivity polymer brushes of poly(styrene-co-4-vinylpyridine). Powder Technol. 2011, 210, 167–174. [Google Scholar] [CrossRef]
- Gopi, D.; Nithiya, S.; Kavitha, L.; Ferreira, J.M.F. Amino acid-assisted synthesis of strontium hydroxyapatite bone cement by a soft solution freezing method. Bull. Mater. Sci. 2012, 35, 1195–1199. [Google Scholar] [CrossRef]
- Vuk, U.; Dragan, P.U. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96B, 152–191. [Google Scholar] [CrossRef]
- Du, X.W.; Chu, Y.; Xing, S.X.; Dong, L.H. Hydrothermal synthesis of calcium hydroxyapatite nanorods in the presence of PVP. J. Mater. Sci. 2009, 44, 6273–6279. [Google Scholar]
- Hao, J.Y.; Liu, Y.; Zhou, S.B.; Li, Z.; Deng, X.M. Investigation of nanocomposites based on semi-interpenetrating network of [l-poly (ε-caprolactone)]/[net-poly (ε-caprolactone)] and hydroxyapatite nanocrystals. Biomaterials 2003, 24, 1531–1539. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Hamada, Y.; Taira, M.; Takahashi, J. Crystallinity and solubility characteristics of hydroxyapatite adsorbed amino acid. Biomaterials 2002, 23, 2241–2247. [Google Scholar] [CrossRef]
- Turki, T.; Aissaa, A.; Bacb, C.G.; Rachdi, F.; Debbabi, M. Study of mixed Ca–Zn hydroxyapatite surface modified by lactic acid. Appl. Surf. Sci. 2012, 258, 6759–6764. [Google Scholar] [CrossRef]
- Sakamoto, K.; Yamaguchi, S.; Nakahira, A.; Kaneno, M.; Okazaki, M.; Ichihara, J.; Tsunawaki, Y.; Elliott, J.C. Shape-controlled synthesis of hydroxyapatite from α-tricalcium bis(orthophosphate) in organic-aqueous binary systems. J. Mater. Sci. 2002, 37, 1033–1041. [Google Scholar] [CrossRef]
- Rong, G.Z.; Deng, M.X.; Deng, C.; Tang, Z.H.; Piao, L.H.; Chen, X.S.; Jing, X.B. Synthesis of poly(ε-caprolactone)-b-poly(γ-benzyl-l-glutamic acid) block copolymer using amino organic calcium catalyst. Biomacromolecules 2003, 4, 1800–1804. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, J.H. New application of poly(butylene succinate) (PBS) based ionomer as biopolymer: A role of ion group for hydroxyapatite (HAp) crystal formation. J. Mater. Sci. 2009, 44, 6398–6403. [Google Scholar] [CrossRef]
- Pan, Y.S.; Xiong, D.S.; Chen, X.L. Mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J. Mater. Sci. 2007, 42, 5129–5134. [Google Scholar] [CrossRef]
- Kandori, K.; Oda, S.; Fukusumi, M.; Marisada, Y. Synthesis of positively charged calcium hydroxyapatite nano-crystals and their adsorption behavior of proteins. Colloids Surf. B Biointerfaces 2009, 73, 140–145. [Google Scholar] [CrossRef]
- Mori, H.; Iwata, M.; Ito, S.; Endo, T. Ring-opening polymerization of γ-benzyl-l-glutamate-N-carboxyanhydride in ionic liquids. Polymer 2007, 48, 5867–5877. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the hydroxyapatite-β-alanine-poly(γ-benzyl-l-glutamates) and hydroxyapatite-β-alanine-poly(l-glutamic acid) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shan, Y.; Qin, Y.; Chuan, Y.; Li, H.; Yuan, M. The Synthesis and Characterization of Hydroxyapatite-β-Alanine Modified by Grafting Polymerization of γ-Benzyl-L-glutamate-N-carboxyanhydride. Molecules 2013, 18, 13979-13991. https://doi.org/10.3390/molecules181113979
Shan Y, Qin Y, Chuan Y, Li H, Yuan M. The Synthesis and Characterization of Hydroxyapatite-β-Alanine Modified by Grafting Polymerization of γ-Benzyl-L-glutamate-N-carboxyanhydride. Molecules. 2013; 18(11):13979-13991. https://doi.org/10.3390/molecules181113979
Chicago/Turabian StyleShan, Yukai, Yuyue Qin, Yongming Chuan, Hongli Li, and Minglong Yuan. 2013. "The Synthesis and Characterization of Hydroxyapatite-β-Alanine Modified by Grafting Polymerization of γ-Benzyl-L-glutamate-N-carboxyanhydride" Molecules 18, no. 11: 13979-13991. https://doi.org/10.3390/molecules181113979