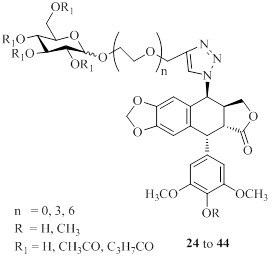
3.4. Click Chemistry-General Procedure
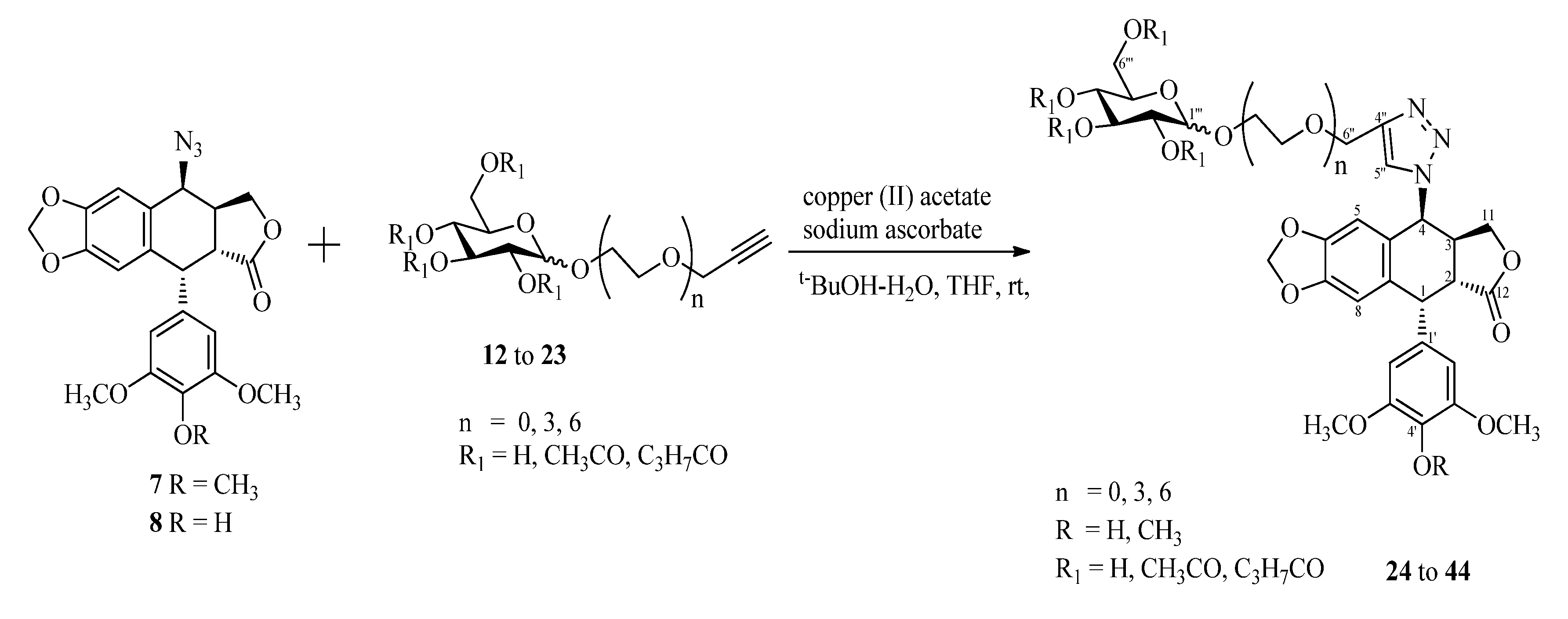
To a solution of a terminal-alkyne 12–23 (0.25 mmol) and 4β-azido-podophyllotoxin analogue (7 or 8, 0.25 mmol) in t−BuOH/H2O (1:2, 1.0 mL) and THF (1.0 mL) at room temperature were added copper (II) acetate (4.6 mg, 0.025 mmol) and sodium ascorbate (1.0 M in H2O, 0.1 mL). The reaction mixture was stirred at room temperature for 31 h until the starting material disappeared as indicated by TLC. Then, the mixture was diluted with water (30 mL) and extracted with ethyl acetate (3 × 20 mL), and the combined organic layer was dried over sodium sulfate. The solvent was evaporated and the residue was purified by column chromatography to afford the cycloaddition product.
4β-[4-(α-d-Glucopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxypodophyllotoxin (24). White amorphous powder, yield 98% (after chromatography with CHCl3/CH3OH, 9:1); mp 168 °C; [α]D24.1: +14.2 (c 0.22, CH3OH); 1H-NMR (CD3OD, 500 MHz): δ 7.84 (s, 1H, C5''-H), 6.65 (s, 1H, C5-H), 6.58 (s, 1H, C8-H), 6.41 (s, 2H, C2', C6'-H), 6.22 (d, 1H, J = 4.7 Hz, C4-H), 5.93 (d, 2H, J = 7.2 Hz, OCH2O), 4.90 (d, 1H, J = 2.8 Hz, C1'''-H), 4.76 (d, 1H, J = 4.0 Hz, C1-H), 4.66 (s, 2H, C6''-CH2); 4.63–4.61 (m, 1H), 4.38–4.34 (m, 1H), 3.85–3.82 (m, 2H), 3.80 (s, 2H, C6'''-CH2), 3.72 (s, 6H, C3'', C5''-OCH3), 3.70 (s, 3H, C4''-OCH3), 3.68–3.58 (m, 2H), 3.45-3.14 (m, 4H); 13C-NMR (CD3OD, 125 MHz): δ 175.8 (C-12), 153.9 (C-3', C-5'), 150.5 (C-7), 149.2 (C-6), 145.6 (C-4''), 138.2 (C-1'), 136.7 (C-9), 134.7 (C-10), 126.8 (C-4'), 125.9 (C-5''), 111.1 (C-5), 109.8 (C-8), 109.3 (C-2', C-6'), 103.3 (OCH2O), 99.7 (C-1'''), 74.9 (C-5'''), 73.9 (C-3'''), 73.3 (C-2'''), 71.7 (C-4'''), 68.9 (C-11), 62.6 (C-6''), 61.5 (C-6'''), 61.1 (4'-OCH3), 59.8 (C-2), 56.6 (3', 5'-OCH3), 44.8 (C-4), 42.4 (C-1), 38.5 (C-3); ESIMS: m/z 680 [M+Na]+, HRESIMS: calcd for C31H35N3O13H [M+H]+ 658.2243, found 658.2223.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-1,2,3-triazol-1-yl]}-4-deoxypodophyllotoxin (25).White amorphous powder, yield 92% (after chromatography with petroleum ether/acetone, 1:1); mp 137 °C; [α]D24.6: +16.3 (c 0.28, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.87 (s, 1H, C5''-H), 6.70 (s, 1H, C5-H), 6.59 (s, 1H, C8-H), 6.41 (s, 2H, C2', C6'-H), 6.25 (d, 1H, J = 4.7 Hz, C4-H), 5.95 (d, 2H, J = 8.3 Hz, OCH2O), 5.38 (t, 1H, J = 9.7 Hz, C3'''-H), 5.18 (d, 1H, J = 3.6 Hz, C1'''-H), 5.03 (t, 1H, J = 8.0 Hz, C4'''-H), 4.79–4.67 (m, 5H, C6''-CH2, C1-H, C2'''-H, C5'''-H), 4.39–4.35 (m, 1H), 4.26–4.20 (m, 1H), 4.11–4.01 (m, 2H), 3.42 (dd, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 3.21–3.16 (m, 1H, C3-H), 2.04 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 1.97 (s, 3H, COCH3), 1.95 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 172.3 (C=O), 171.7 (C=O), 171.5 (C=O), 171.3 (C=O), 153.9 (C-3', C-5'), 150.5 (C-7), 149.3 (C-4''), 144.5 (C-6), 138.3 (C-1'), 136.7 (C-9), 134.7 (C-10), 126.9 (C-4'), 126.4 (C-5''), 111.2 (C-5), 109.9 (C-8), 109.4 (C-2', C-6'), 103.3 (OCH2O), 95.6 (C-1'''), 72.2 (C-5'''), 71.2 (C-3'''), 69.8 (C-2'''), 68.9 (C-11), 68.8 (C-4'''), 63.1 (C-6''), 61.3 (C-6'''), 61.1 (4'-OCH3), 59.8 (C-2), 56.6 (3', 5'-OCH3), 44.9 (C-4), 42.5 (C-1), 38.5 (C-3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3); ESIMS: m/z 848 [M+Na]+, HRESIMS: calcd for C39H43N3O17Na [M+Na]+ 848.2485, found 848.2472.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-1,2,3-triazol-1-yl]}-4-deoxypodophyllotoxin (26). White amorphous powder, yield 98% (after chromatography with petroleum ether/acetone, 1:1); mp 101 °C; [α]D25.1: −41.3 (c 0.23, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.74 (s, 1H, C5''-H), 6.67 (s, 1H, C5-H), 6.59 (s, 1H, C8-H), 6.41 (s, 2H, C2', C6'-H), 6.24 (d, 1H, J = 4.5 Hz, C4-H), 5.95 (d, 2H, J = 5.6 Hz, OCH2O), 5.26 (t, 1H, J = 9.4 Hz, C3'''-H), 5.08 (t, 1H, J = 12.0 Hz, C4'''-H), 4.93–4.73 (m, 6H, C1'''-H, C6''-CH2, C1-H, C2'''-H, C5'''-H), 4.33-3.89 (m, 4H), 3.72 (s, 6H, C3'', C5''-OCH3), 3.70 (s, 3H, C4''-OCH3), 3.40 (dd, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 3.14–3.10 (m, 1H, C3-H), 2.31–2.13 (m, 2H, COCH2), 2.31–2.13 (m, 2H, COCH2), 2.31–2.13 (m, 2H, COCH2), 2.31–2.13 (m, 2H, COCH2), 1.63–1.47 (m, 2H, CH2CH3), 1.63–1.47 (m, 2H, CH2CH3), 1.63–1.47 (m, 2H, CH2CH3), 1.63-1.47 (m, 2H, CH2CH3), 0.92 (t, 3H, J = 4.1 Hz, CH2CH3), 0.90 (t, 3H, J = 4.1 Hz, CH2CH3), 0.87 (t, 3H, J = 4.1 Hz, CH2CH3), 0.83 (t, 3H, J = 4.1 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 175.6 (C-12), 174.6 (C=O), 173.8 (C=O), 173.5 (C=O), 173.5 (C=O), 153.9 (C-3', C-5'), 150.5 (C-7), 149.2 (C-4''), 145.3 (C-6), 138.3 (C-1'), 136.6 (C-9), 134.7 (C-10), 126.9 (C-4'), 125.9 (C-5''), 111.2 (C-5), 109.8 (C-8), 109.4 (C-2', C-6'), 103.3 (OCH2O), 101.1 (C-1'''), 73.8 (C-5'''), 73.1 (C-3'''), 72.4 (C-2'''), 69.4 (C-4'''), 68.8 (C-11), 63.3 (C-6''), 62.7 (C-6'''), 61.1 (4'-OCH3), 59.8 (C-2), 56.6 (3', 5'-OCH3), 44.9 (C-4), 42.5 (C-1), 38.5 (C-3), 36.8 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.2 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3); ESIMS: m/z 960 [M+Na]+, HRESIMS: calcd for C47H59N3O17H [M+H]+ 938.3917, found 938.3877.
4β-[4-(α-d-Glucopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxy-4'-demethylpodophyllotoxin (27). White amorphous powder, yield 98% (after chromatography with CHCl3/CH3OH, 9:1); mp 227 °C; [α]D24.9: +1.0 (c 0.30, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.87 (s, 1H, C5''-H), 6.68 (s, 1H, C5-H), 6.64 (s, 1H, C8-H), 6.38 (s, 2H, C2', C6'-H), 6.26 (d, 1H, J = 4.8 Hz, C4-H), 5.97 (d, 2H, J = 9.7 Hz, OCH2O), 4.84–4.42 (m, 6H, C1'''-H, C1-H, C6''-CH2, C11-CH2), 3.83–3.78 (m, 3H, C3'''-H, C6'''-CH2), 3.75 (s, 6H, C3'', C5''-OCH3), 3.67–3.60 (m, 3H), 3.43–3.34 (m, 1H, C2-H), 3.26–3.18 (m, 1H, C3-H); 13C-NMR (CD3OD, 100 MHz): δ 176.5 (C-12), 150.7 (C-7), 149.6 (C-6), 148.1 (C-3', C-5'), 145.7 (C-4''), 135.9 (C-1'), 135.2 (C-9), 131.5 (C-10), 126.8 (C-4'), 126.2 (C-5''), 111.3 (C-5), 109.2 (C-8), 109.9 (C-2', C-6'), 103.4 (OCH2O), 99.8 (C-1'''), 74.9 (C-5'''), 74.0 (C-3'''), 73.4 (C-2'''), 71.7 (C-4'''), 69.2 (C-11), 62.7 (C-6''), 61.5 (C-6'''), 60.1 (C-2), 56.9 (3', 5'-OCH3), 44.8 (C-4), 42.8 (C-1), 38.6 (C-3); ESIMS: m/z 667 [M+Na]+, HRESIMS: calcd for C30H34N3O13Na [M+Na]+ 667.1984, found 667.1961.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-1,2,3-triazol-1-yl]}-4-deoxy-4'-demethylpodophyllotoxin (28). White amorphous powder, yield 94% (after chromatography with petroleum ether/acetone, 1:1); mp 128 °C; [α]D24.8: +27.0 (c 0.14, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.24 (s, 1H, C5''-H), 6.60 (s, 1H, C5-H), 6.26 (s, 1H, C8-H), 6.58 (s, 2H, C2', C6'-H), 6.02–5.91 (m, 3H, C4-H, OCH2O), 5.42 (t, 1H, J = 9.9 Hz, C3'''-H), 5.24 (d, 1H, J = 3.5 Hz, C1'''-H), 5.04 (t, 1H, J = 9.4 Hz, C4'''-H), 4.85 (s, 2H, C6''-CH2), 4.81–4.76 (m, 2H, C2'''-H, C5'''-H), 4.67 (d, 1H, J = 4.2 Hz, C1-H), 4.26–4.00 (m, 4H, C6'''-CH2, C11-CH2), 3.78 (s, 6H, C3'', C5''-OCH3), 3.57-3.46 (m, 1H, C3-H), 3.25 (dd, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 2.03 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 1.98 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 176.0 (C-12), 172.3 (C=O), 171.7 (C=O), 171.5 (C=O), 171.2 (C=O), 149.7 (C-7), 149.1(C-6), 148.6 (C-3', C-5'), 144.7 (C-4''), 135.5 (C-1'), 134.3 (C-9), 131.6 (C-10), 129.1 (C-4'), 126.2 (C-5''), 110.9 (C-5), 109.2 (C-2', C-6'), 107.2 (C-8), 103.1 (OCH2O), 96.2 (C-1'''), 72.1 (C-5'''), 71.3 (C-3'''), 71.3 (C-11), 69.8 (C-2'''), 68.9 (C-4'''), 63.9 (C-2), 62.9 (C-6''), 61.7 (C-6'''), 56.8 (3', 5'-OCH3), 46.6 (C-4), 45.1 (C-1), 39.9 (C-3), 20.6 (COCH3), 20.6 (COCH3), 20.6 (COCH3), 20.5 (COCH3); ESIMS: m/z 834 [M+Na]+, HRESIMS: calcd for C38H41N3O17Na [M+H]+ 812.2509, found 812.2480.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-1,2,3-triazol-1-yl]}-4-deoxy-4'-demethylpodophyllotoxin (29). White amorphous powder, yield 96% (after chromatography with petroleum ether/acetone, 1:1); mp 102 °C; [α]D24.7: +25.9 (c 0.29, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.21 (s, 1H, C5''-H), 6.57 (s, 3H, C2', C6'-H, C5-H), 6.18 (s, 1H, C8-H), 5.91 (d, 2H, J = 6.0 Hz, OCH2O), 5.93–5.88 (m, 3H, OCH2O, C4-H), 5.47 (t, 1H, J = 9.7 Hz, C3'''-H), 5.25 (d, 1H, J = 3.0 Hz, C1'''-H), 5.11 (t, 1H, J = 9.7 Hz, C4'''-H), 4.84 (s, 2H, C6''-CH2), 4.81 (d, 1H, J = 4.0 Hz, C1-H), 4.77–4.61 (m, 2H), 4.23–4.03 (m, 4H, C6'''-CH2, C11-CH2), 3.55–3.44 (m, 1H, C3-H), 3.06 (d, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 2.29–2.14 (m, 2H, COCH2), 2.29-2.14 (m, 2H, COCH2), 2.29–2.14 (m, 2H, COCH2), 2.29–2.14 (m, 2H, COCH2), 1.62–1.47 (m, 2H, CH2CH3), 1.62–1.47 (m, 2H, CH2CH3), 1.62–1.47 (m, 2H, CH2CH3), 1.62–1.47 (m, 2H, CH2CH3), 0.90 (t, 3H, J = 7.8 Hz, CH2CH3), 0.88 (t, 3H, J = 7.8 Hz, CH2CH3), 0.86 (t, 3H, J = 7.8 Hz, CH2CH3), 0.81 (t, 3H, J = 7.8 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 174.7 (C=O), 174.0 (C=O), 173.9 (C=O), 173.5 (C=O), 149.5 (C-7), 149.0 (C-6), 148.5 (C-3', C-5'), 144.6 (C-4''), 135.4 (C-1'), 134.3 (C-9), 131.7 (C-10), 129.0 (C-4'), 126.2 (C-5''), 111.0 (C-5), 109.2 (C-2', C-6'), 107.3 (C-8), 103.1 (OCH2O), 96.3 (C-1'''), 72.0 (C-5'''), 71.1 (C-3'''), 69.5 (C-2'''), 69.1 (C-4'''), 71.3 (C-11), 63.8 (C-2), 62.8 (C-6''), 61.7 (C-6'''), 56.9 (3', 5'-OCH3), 46.4 (C-4), 45.0 (C-1), 39.8 (C-3), 36.9 (COCH2), 36.9 (COCH2), 36.8 (COCH2), 36.7 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3); ESIMS: m/z 946 [M+Na]+, HRESIMS: calcd for C46H57N3O17H [M+H]+ 924.3761, found 924.3722.
4β-{4-[1-(α-d-Glucopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (30). White amorphous powder, yield 91% (after chromatography with CHCl3/CH3OH, 9:1); [α]D25.0: +0.5 (c 0.15, CH3OH); 1H-NMR (CD3OD, 600 MHz): δ 7.84 (s, 1H, C5''-H), 6.70 (s, 1H, C5-H), 6.63 (s, 1H, C8-H), 6.41 (s, 2H, C2', C6'-CH), 6.27 (d, 1H, J = 3.2 Hz, C4-H), 5.98 (d, 2H, J = 4.8 Hz, OCH2O), 4.82 (d, 1H, J = 3.5 Hz, C1'''-H), 4.81 (d, 1H, J = 4.0 Hz, C1-H), 4.62 (s, 2H, C6''-CH2), 3.79–3.86 (m, 4H), 3.74 (s, 6H, C3'', C5''-OCH3), 3.72 (s, 3H, C4''-OCH3), 3.69–3.67 (m, 6H), 3.65–3.61 (m, 6H), 3.47–3.44 (m, 2H), 3.38–3.36 (m, 2H), 3.30–3.27 (m, 1H, C3-H), 3.17–3.14 (m, 1H, C2-H); 13C-NMR (CD3OD, 150 MHz): δ 176.0 (C-12), 154.1 (C-3', C-5'), 150.7 (C-7), 149.5 (C-6), 146.1 (C-4''), 138.4 (C-1'), 136.9 (C-9), 134.9 (C-10), 127.1 (C-4'), 126.1 (C-5''), 111.3 (C-5), 110.0 (C-8), 109.4 (C-2', C-6'), 103.5 (OCH2O), 100.5 (C-1'''), 75.3 (C-5'''), 73.8 (C-3'''), 73.8 (C-2'''), 71.9 (C-4'''), 71.6, 71.6, 71.6, 71.5, 71.1 (C-11), 69.1, 68.3, 65.1 (C-6''), 62.8 (C-6'''), 61.2 (4'-OCH3), 59.9 (C-2), 56.8 (3', 5'-OCH3), 45.1 (C-4), 42.7 (C-1), 38.7 (C-3); ESIMS: m/z 812 [M+Na]+, HRESIMS: calcd for C37H47N3O16Na [M+Na]+ 812.2849, found 812.2822.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (31). White amorphous powder, yield 94% (after chromatography with petroleum ether/acetone, 1:1); [α]D25.0: +14.3 (c 0.20, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.18 (s, 1H, C5''-H), 6.62 (s, 2H, C2', C6'-CH), 6.40 (s, 1H, C5-H), 6.25 (s, 1H, C8-H), 5.97–5.92 (m, 3H, C4-H OCH2O), 5.43 (t, 1H, J = 12.0 Hz, C3'''-H), 5.10 (d, 1H, J = 3.1 Hz, C1'''-H), 5.02 (t, 1H, J = 12.0 Hz, C4'''-H), 4.84–4.76 (m, 2H), 4.61 (s, 2H, C6''-CH2), 4.25–4.06 (m, 4H, C6'''-CH2, C11-CH2), 3.78 (s, 6H, C3'', C5''-OCH3), 3.72 (s, 3H, C4''-OCH3), 3.71–3.60 (m, 12H), 3.25 (dd, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 3.19–3.14 (m, 1H, C3-H), 2.02 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 1.98 (s, 3H, COCH3), 1.96 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 172.3 (C=O), 171.6 (C=O), 171.6 (C=O), 171.3 (C=O), 153.9 (C-3', C-5'), 150.5 (C-4''), 149.7 (C-7), 149.3 (C-6), 136.9 (C-1'), 134.7 (C-9), 133.9 (C-10),127.0 (C-4'), 125.6 (C-5''), 110.9 (C-5), 109.9 (C-8), 109.4 (C-2', C-6'), 103.2 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.7, 71.6, 71.6, 71.5, 71.5, 71.2, 71.0, 71.0, 69.9 (C-2'''), 68.7 (C-11), 68.5 (C-4'''), 64.6 (C-6''), 63.2 (C-6'''), 61.1 (4'-OCH3), 56.7 (C-2), 56.6 (3', 5'-OCH3), 45.2 (C-4), 45.5 (C-1), 39.9 (C-3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3); ESIMS: m/z 980 [M+Na]+, HRESIMS: calcd for C45H55N3O20H [M+H]+ 958.3452, found 958.3403.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (32). White amorphous powder, yield 90% (after chromatography with petroleum ether/acetone, 1:1); [α]D24.0: +19.3 (c 0.14, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.17 (s, 1H, C5''-H), 6.61 (s, 2H, C2', C6'-H), 6.40 (s, 1H, C5-H), 6.25 (s, 1H, C8-H), 5.97–5.90 (m, 3H, C4-H, OCH2O), 5.48 (t, 1H, J = 12.0 Hz, C3'''-H), 5.11–5.05 (m, 2H, C1'''-H, C4'''-H), 4.87–4.83 (m, 2H), 4.76 (d, 1H, J = 4.0 Hz, C1-H), 4.60 (s, 2H, C6''-CH2), 4.23–4.08 (m, 4H, C6'''-CH2, C11-CH2), 3.71 (s, 6H, C3'', C5''-OCH3), 3.70 (s, 3H, C4''-OCH3), 3.70–3.59 (m, 12H), 3.22–3.12 (m, 2H, C2-H, C3-H), 2.31–2.19 (m, 2H, COCH2), 2.31–2.19 (m, 2H, COCH2), 2.31–2.19 (m, 2H, COCH2), 2.31–2.19 (m, 2H, COCH2), 1.63–1.53 (m, 2H, CH2CH3), 1.63–1.53 (m, 2H, CH2CH3), 1.63-1.53 (m, 2H, CH2CH3), 1.63-1.53 (m, 2H, CH2CH3), 0.92 (t, 3H, J = 4.0 Hz, CH2CH3), 0.90 (t, 3H, J = 4.0 Hz, CH2CH3), 0.88 (t, 3H, J = 4.0 Hz, CH2CH3), 0.86 (t, 3H, J = 4.0 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 175.7 (C-12), 174.7 (C=O), 174.1 (C=O), 174.0 (C=O), 173.6 (C=O), 153.9 (C-3', C-5'), 150.5 (C-4''), 149.6 (C-7), 146.2 (C-6), 137.9 (C-9), 136.9 (C-10), 133.9 (C-1'), 127.0 (C-4'), 125.6 (C-5''), 110.9 (C-5), 109.8 (C-8), 109.4 (C-2', C-6'), 103.2 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.7, 71.6, 71.6, 71.6, 71.2 (C-3'''), 69.6 (C-2'''), 68.7 (C-4'''), 68.6 (C-11), 65.2 (C-6''), 63.0 (C-6'''), 61.1 (4'-OCH3), 56.7 (C-2), 56.7 (3', 5'-OCH3), 45.2 (C-4), 42.5 (C-1), 39.9 (C-3), 36.8 (COCH2), 36.8 (COCH2), 36.8 (COCH2), 36.8 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3), 14.1 (CH2CH3); ESIMS: m/z 1092 [M+Na]+, HRESIMS: calcd for C53H71N3O20H [M+H]+ 1070.4704, found 1070.4658.
4β-{4-[1-(α-d-Glucopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (33). White amorphous powder, yield 93% (after chromatography with CHCl3/CH3OH, 9:1); [α]D25.3: −37.2 (c 0.11, CH3OH); 1H-NMR (CD3OD, 600 MHz): δ 7.83 (s, 1H, C5''-H), 6.69 (s, 1H, C5-H), 6.66 (s, 1H, C8-H), 6.38 (s, 2H, C2', C6'-H), 6.27 (d, 1H, J = 3.2 Hz, C4-H), 5.99 (d, 2H, J = 5.6 Hz, OCH2O), 4.82 (d, 1H, J = 4.0 Hz, C1'''-H), 4.78 (d, 1H, J = 4.0 Hz, C1-H), 4.63 (s, 2H, C6''-CH2), 3.86–3.79 (m, 4H, C6'''-CH2, C11-CH2), 3.75 (s, 6H, C3'', C5''-OCH3), 3.69–3.61 (m, 12H), 3.44–3.40 (m, 2H), 3.38–3.36 (m, 2H), 3.30–3.26 (m, 2H), 3.18–3.14 (m, 2H, C2-H, C3-H); 13C-NMR (CD3OD, 150 MHz): δ 176.2 (C-12), 150.7 (C-7), 149.4 (C-4''), 148.8 (C-3', C-5'), 146.1 (C-6), 136.1 (C-1'), 135.3 (C-9), 131.5 (C-10), 127.1 (C-4'), 126.1 (C-5''), 111.4 (C-5), 109.9 (C-8), 109.4 (C-2', C-6'), 103.4 (OCH2O), 100.5 (C-1'''), 75.3 (C-5'''), 73.8 (C-3'''), 73.8 (C-2'''), 72.0 (C-4'''), 71.7, 71.6, 71.6, 71.5 (C-11), 71.1, 69.1, 68.3, 65.1 (C-6''), 62.8 (C-6'''), 60.0 (C-2), 56.9 (3', 5'-OCH3), 44.9 (C-4), 42.8 (C-1), 38.7 (C-3); ESIMS: m/z 798 [M+Na]+, HRESIMS: calcd for C36H45N3O16Na [M+Na]+ 798.2692, found 798.2669.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (34). White amorphous powder, yield 94% (after chromatography with petroleum ether/acetone, 1:1); [α]D23.8: +14.4 (c 0.23, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.18 (s, 1H, C5''-H), 6.61 (s, 1H, C5-H), 6.58 (s, 2H, C2', C6'-H), 6.25 (s, 1H, C8-H), 6.03–5.93 (m, 3H, C4-H, OCH2O), 5.42 (d, 1H, J = 8.0 Hz, C3'''-H), 5.10 (d, 1H, J = 2.8 Hz, C1'''-H), 5.02 (t, 1H, J = 8.0 Hz, C4'''-H), 4.83–4.81 (m, 2H), 4.70–4.67 (m, 3H, C6''-CH2, C1-H), 4.25–4.07 (m, 4H, C6'''-CH2, C11-CH2), 3.79 (s, 6H, C3'', C5''-OCH3), 3.72–3.63 (m, 12H), 3.57–3.45 (m, 1H, C3-H), 3.26 (dd, 1H, J = 4.0 Hz, 16.0 Hz, C2-H), 2.02 (s, 3H, COCH3), 2.01 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 1.96 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 176.0 (C-12), 172.4 (C=O), 171.8 (C=O), 171.7 (C=O), 171.7 (C=O), 149.7 (C-7), 149.1 (C-6), 148.6 (C-3', C-5'), 145.8 (C-4''), 135.6 (C-1'), 134.3 (C-9), 131.6 (C-10), 129.2 (C-4'), 125.6 (C-5''), 110.9 (C-5), 109.2 (C-2', C-6'), 107.2 (C-8), 103.1 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.6, 71.6,71.5 (C-3'''), 71.2, 71.0, 70.0 (C-2'''), 68.7 (C-11), 68.5 (C-4'''), 65.1 (C-6''), 63.9 (C-2), 63.2 (C-6'''), 56.8 (3', 5'-OCH3), 46.6 (C-4), 45.1 (C-1), 39.9 (C-3), 20.7 (COCH3), 20.6 (COCH3), 20.6 (COCH3), 20.6 (COCH3); ESIMS: m/z 966 [M+Na]+, HRESIMS: calcd for C44H53N3O20H [M+H]+ 944.3295, found 944.3249.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (35). White amorphous powder, yield 92% (after chromatography with petroleum ether/acetone, 1:1); [α]D25.0: +22.4 (c 0.24, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.18 (s, 1H, C5''-H), 6.61 (s, 1H, C5-H), 6.59 (s, 2H, C2'-H, C6'-H), 6.26 (s, 1H, C8-H), 6.04–5.94 (m, 3H, C4-H, OCH2O), 5.47 (t, 1H, J = 12.0 Hz, C3'''-H), 5.11 (d, 2H, J = 3.7 Hz, C1'''-H), 5.06 (t, 1H, J = 8.0 Hz, C4'''-H), 4.85–4.81 (m, 2H), 4.71–4.69 (m, 3H, C6''-CH2, C1-H), 4.23–4.09 (m, 4H, C6'''-CH2, C11-CH2), 3.80 (s, 6H, C3'', C5''-OCH3), 3.72–3.64 (m, 12H), 3.55–3.49 (m, 1H, C3-H), 3.28–3.26 (m, 1H, C2-H), 2.32–2.19 (m, 2H, COCH2), 2.32–2.19 (m, 2H, COCH2), 2.32–2.19 (m, 2H, COCH2), 2.32–2.19 (m, 2H, COCH2), 1.62–1.53 (m, 2H, CH2CH3), 1.62–1.53 (m, 2H, CH2CH3), 1.62–1.53 (m, 2H, CH2CH3), 1.62–1.53 (m, 2H, CH2CH3), 0.93 (t, 3H, J = 4.0 Hz, CH2CH3), 0.91 (t, 3H, J = 4.0 Hz, CH2CH3), 0.89 (t, 3H, J = 4.0 Hz, CH2CH3), 0.87 (t, 3H, J = 4.0 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 176.0 (C-12), 174.8 (C=O), 174.1 (C=O), 174.1 (C=O), 173.6 (C=O), 149.7 (C-7), 149.1 (C-6), 148.6 (C-3', C-5'), 134.3 (C-1'), 131.5 (C-9), 131.5 (C-10), 127.2 (C-4'), 125.5 (C-5''), 110.9 (C-5), 109.2 (C-2', C-6'), 107.2 (C-8), 103.1 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.6, 71.6, 71.3, 71.2 (C-3'''), 71.0, 69.6 (C-2'''), 68.7 (C-4'''), 68.7 (C-11), 65.1 (C-6''), 63.9 (C-2), 62.9 (C-6'''), 56.8 (3', 5'-OCH3), 46.6 (C-4), 45.2 (C-1), 39.9 (C-3), 36.9 (COCH2), 36.8 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.2 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3); ESIMS: m/z 1078 [M+Na]+, HRESIMS: calcd for C52H69N3O20H [M+H]+ 1056.4547, found 1056.4484.
4β-{4-[1-(α-d-Glucopyranosyloxymethyl)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (36). White amorphous powder, yield 91% (after chromatography with CHCl3/CH3OH, 9:1); [α]D24.3: −2.5 (c 0.17, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.82 (s, 1H, C5''-H), 6.69 (s, 1H, C5-H), 6.64 (s, 1H, C8-H), 6.41 (s, 2H, C2', C6'-H), 6.26 (d, 1H, J = 4.8 Hz, C4-H), 5.98 (d, 2H, J = 5.5 Hz, OCH2O), 4.82–4.80 (m, 2H, C1'''-H, C1-H), 4.62 (s, 2H, C6''-CH2), 3.87–3.78 (m, 4H, C6'''-CH2, C11-CH2), 3.74 (s, 6H, C3'', C5''-OCH3), 3.72 (s, 3H, C4''-OCH3), 3.68–3.60 (m, 24H), 3.47–3.42 (m, 2H), 3.38–3.35 (m, 2H), 3.28–3.25 (m, 1H), 3.18–3.13 (m, 1H); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 153.9 (C-3', C-5'), 150.6 (C-7), 149.3 (C-6), 148.1 (C-4''), 138.3 (C-1'), 136.7 (C-9), 134.7 (C-10), 126.9 (C-4'), 125.9 (C-5''), 111.2 (C-5), 109.8 (C-8), 109.3 (C-2', C-6'), 103.3 (OCH2O), 100.3 (C-1'''), 75.2 (C-5'''), 73.7 (C-3'''), 73.7 (C-2'''), 71.8 (C-4'''), 71.5, 71.5, 71.5, 71.3, 70.9 (C-11), 68.9, 68.1, 65.0 (C-6''), 62.7 (C-6'''), 61.1 (4'-OCH3), 59.8 (C-2), 56.7 (3', 5'-OCH3), 44.9 (C-4), 42.5 (C-1), 38.6 (C-3); ESIMS: m/z 944 [M+Na]+, HRESIMS: calcd for C43H59N3O19Na [M+Na]+ 944.3635, found 944.3622.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (37). White amorphous powder, yield 91% (after chromatography with petroleum ether/acetone, 1:1); [α]D23.9: −24.9 (c 0.22, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.21 (s, 1H, C5′′-H), 6.62, (s, 2H, C2′, C6′-H), 6.60 (s, 1H, C5-H), 6.25 (s, 1H, C8-H), 6.05–5.94 (m, 3H, C4-H, OCH2O), 5.43 (t, 1H, J = 12.0 Hz, C3'''-H), 5.11 (d, 1H, J = 3.5 Hz, C1'''-H), 5.02 (t, 1H, J = 12.0 Hz, C4'''-H), 4.85–4.80 (m, 3H), 4.70 (s, 2H, C6''-CH2), 4.26–4.03 (m, 4H, C6'''-CH2, C11-CH2), 3.79 (s, 6H, C3'', C5''-OCH3), 3.73 (s, 3H, C4''-OCH3), 3.65–3.59 (m, 24H), 3.55-3.45 (m, 2H, C2-H, C3-H), 2.04 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 1.97 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 172.3 (C=O), 171.7 (C=O), 171.7 (C=O), 171.3 (C=O), 153.9 (C-3', C-5'), 153.9 (C-7), 149.7 (C-6), 149.2 (C-4''), 137.9 (C-1'), 137.0 (C-9), 133.9 (C-10), 129.3 (C-4'), 125.7 (C-5''), 110.9 (C-5), 109.3 (C-2', C-6'), 107.2 (C-8), 103.2 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.6, 71.5, 71.5, 71.2 (C-3'''), 71.0 (C-2'''), 70.0, 68.7 (C-4'''), 68.5 (C-11), 65.1 (C-6''), 63.8 (C-2), 63.2 (C-6'''), 61.1 (4'-OCH3), 56.7 (3', 5'-OCH3), 45.5 (C-4), 45.3 (C-1), 39.9 (C-3), 20.7 (COCH3), 20.6 (COCH3), 20.6 (COCH3), 20.6 (COCH3); ESIMS: m/z 1090 [M+H]+, HRESIMS: calcd for C51H67N3O23H [M+H]+ 1090.4238, found 1090.4177.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (38). White amorphous powder, yield 91% (after chromatography with petroleum ether/acetone, 1:1); [α]D23.7: +17.4 (c 0.20, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.20 (s, 1H, C5''-H), 6.62 (s, 2H, C2', C6'-H), 6.59 (s, 1H, C5-H), 6.25 (s, 1H, C8-H), 6.04–5.93 (m, 3H, C4-H, OCH2O), 5.48 (t, 1H, J = 8.0 Hz, C3'''-H), 5.11–5.04 (m, 2H, C1'''-H, C4'''-H), 4.85–4.81 (m, 2H), 4.72–4.70 (m, 3H, C6''-CH2, C1-H), 4.23–4.09 (m, 4H, C6'''-CH2, C11-CH2), 3.79 (s, 6H, C3'', C5''-OCH3), 3.72 (s, 3H, C4''-OCH3), 3.65–3.61 (m, 24H), 3.27–3.26 (m, 1H), 3.18–3.13 (m, 1H), 2.33–2.20 (m, 2H, COCH2), 2.33–2.20 (m, 2H, COCH2), 2.33–2.20 (m, 2H, COCH2), 2.33-2.20 (m, 2H, COCH2), 1.65–1.53 (m, 2H, CH2CH3), 1.65–1.53 (m, 2H, CH2CH3), 1.65–1.53 (m, 2H, CH2CH3), 1.65–1.53 (m, 2H, CH2CH3), 0.94 (t, 3H, J = 4.0 Hz, CH2CH3), 0.91 (t, 3H, J = 4.0 Hz, CH2CH3), 0.90 (t, 3H, J = 4.0 Hz, CH2CH3), 0.88 (t, 3H, J = 4.0 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 175.8 (C-12), 174.7 (C=O), 174.1 (C=O), 174.0 (C=O), 173.6 (C=O), 153.9 (C-3', C-5'), 149.7 (C-7), 149.2 (C-6), 146.3 (C-4''), 137.9 (C-1'), 136.9 (C-9), 133.9 (C-10), 129.3 (C-4'), 125.8 (C-5''), 110.9 (C-5), 109.4 (C-2', C-6'), 107.3 (C-8), 103.2 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.6, 71.5, 71.2, 71.0 (C-3'''), 69.6 (C-2'''), 68.7 (C-11), 68.6 (C-4'''), 65.2 (C-6''), 63.8 (C-2), 63.0 (C-6'''), 61.1 (4'-OCH3), 56.7 (3', 5'-OCH3), 46.5 (C-4), 45.2 (C-1), 39.9 (C-3), 36.9 (COCH2), 36.8 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3); ESIMS: m/z 1224 [M+Na]+, HRESIMS: calcd for C59H83N3O23H [M+H]+ 1202.5490, found 1202.5423.
4β-{4-[1-(α-d-Glucopyranosyloxymethyl)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (39). White amorphous powder, yield 93% (after chromatography with CHCl3/CH3OH, 9:1); mp 158 °C; [α]D25.4: −4.0 (c 0.11, CH3OH); 1H-NMR (CD3OD, 600 MHz): δ 7.82 (s, 1H, C5''-H), 6.69 (s, 1H, C5-H), 6.66 (s, 1H, C8-H), 6.38 (s, 2H, C2', C6'-H), 6.27 (d, 1H, J = 3.6 Hz, C4-H), 5.98 (d, 2H, J = 5.6 Hz, OCH2O), 4.82 (d, 1H, J = 2.4 Hz, C1'''-H), 4.78 (d, 1H, J = 3.6 Hz, C1-H), 4.64 (s, 2H, C6''-CH2), 3.85–3.80 (m, 4H, C6'''-CH2, C11-CH2), 3.75 (s, 6H, C3'', C5''-OCH3), 3.69–3.60 (m, 24H), 3.42–3.40 (m, 2H), 3.38–3.36 (m, 1H), 3.18–3.14 (m ,2H, C2-H, C3-H); 13C-NMR (CD3OD, 150 MHz): δ 176.2 (C-12), 150.7 (C-7), 149.4 (C-6), 148.8 (C-3', C-5'), 135.2 (C-4''), 132.5 (C-1'), 131.5 (C-9), 130.0 (C-10), 127.1 (C-4'), 126.1 (C-5''), 111.4 (C-5), 109.9 (C-8), 109.4 (C-2', C-6'), 103.4 (OCH2O), 100.4 (C-1'''), 75.3 (C-5'''), 73.9 (C-3'''), 73.9 (C-2'''), 71.9 (C-4'''), 71.7, 71.7, 71.6, 71.5, 71.1, 70.9 (C-11), 69.2, 68.2, 65.1 (C-6''), 62.3 (C-6'''), 60.0 (C-2), 56.9 (3', 5'-OCH3), 44.9 (C-4), 42.9 (C-1), 68.7 (C-3); ESIMS: m/z 930 [M+Na]+, HRESIMS: calcd for C42H57N3O19Na [M+Na]+ 930.3478, found 930.3462.
4β-{4-[1-(2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyloxy)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (40). White amorphous powder, yield 90% (after chromatography with petroleum ether/acetone, 1:1); [α]D25.0: +25.1 (c 0.23, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.18 (s, 1H, C5''-H), 6.60 (s, 1H, C5-H), 3.58 (s, 2H, C2', C6'-H), 6.23 (s, 1H, C8-H), 6.01–5.92 (m, 3H, C4-H, OCH2O), 5.43 (t, 1H, J = 12.0 Hz, C3'''-H), 5.11 (d, 1H, J = 4.0 Hz, C1'''-H), 5.02 (t, 1H, J = 12.0 Hz, C4'''-H), 4.69 (s, 2H, C6''-CH2), 4.65 (d, 1H, J = 4.0 Hz, C1-H), 4.30–4.07 (m, 4H, C6'''-CH2, C11-CH2), 3.78 (s, 6H, C3'', C5''-OCH3), 3.69–3.59 (m, 24H), 3.44–3.41 (m, 1H, C3-H), 3.21 (dd, 1H, J = 4.0 Hz, 12.0 Hz, C4-H), 2.04 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 1.97 (s, 3H, COCH3); 13C-NMR (CD3OD, 100 MHz): δ 174.4 (C-12), 170.8 (C=O), 170.2 (C=O), 170.2 (C=O), 169.8 (C=O), 148.1 (C-7), 147.6 (C-6), 147.1 (C-3', C-5'), 144.6 (C-4''), 134.0 (C-1'), 132.7 (C-9), 130.1 (C-10), 128.3 (C-4'), 127.7 (C-5''), 109.4 (C-5), 107.6 (C-2', C-6'), 105.7 (C-8), 101.6 (OCH2O), 95.5 (C-1'''), 72.1, 71.6, 71.6, 71.5, 71.2, 71.0 (C-5'''), 70.0 (C-3'''), 68.4 (C-2'''), 67.2 (C-11), 66.9 (C-4'''), 63.5 (C-6''), 62.3 (C-2), 61.6 (C-6'''), 55.3 (3', 5'-OCH3), 45.0 (C-4), 43.6 (C-1), 38.3 (C-3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3); ESIMS: m/z 1098 [M+Na]+, HRESIMS: calcd for C50H65N3O23H [M+H]+ 1076.4082, found 1076.4031.
4β-{4-[1-(2,3,4,6-Tetra-O-butyryl-α-d-glucopyranosyloxy)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (41). White amorphous powder, yield 97% (after chromatography with petroleum ether/acetone, 1:1); [α]D24.6: −3.1 (c 0.29, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 8.18 (s, 1H, C5''-H), 6.62 (s, 1H, C5-H), 6.59 (s, 2H, C2', C6'-H), 6.25 (s, 1H, C8-H), 5.95–5.84 (m, 3H, C4-H, OCH2O), 5.37 (t, 1H, J = 8.0 Hz, C3'''-H), 5.02 (d, 2H, J = 4.0 Hz, C1'''-H), 4.97 (t, 1H, J = 12.0 Hz, C4'''-H), 4.61–4.59 (m, 3H, C6''-CH2, C1-H), 4.13–4.00 (m, 4H, C6'''-CH2, C11-CH2), 3.80 (s, 6H, C3'', C5''-OCH3), 3.53–3.51 (m, 24H), 3.45–3.39 (m, 2H, C2-H, C3-H), 2.24–2.10 (m, 8H), 1.56–1.43 (m, 8H), 0.85 (t, 3H, J = 4.0 Hz, CH2CH3), 0.82 (t, 3H, J = 4.0 Hz, CH2CH3), 0.80 (t, 3H, J = 4.0 Hz, CH2CH3), 0.79 (t, 3H, J = 4.0 Hz, CH2CH3); 13C-NMR (CD3OD, 100 MHz): δ 176.0 (C-12), 174.8 (C=O), 174.0 (C=O), 174.0 (C=O), 173.6 (C=O), 149.7 (C-7), 149.1 (C-6), 148.6 (C-3', C-5'), 146.2 (C-4''), 135.6 (C-1'), 134.3 (C-9), 131.6 (C-10), 129.2 (C-4'), 125.6 (C-5''), 110.0 (C-5), 109.1 (C-2', C-6'), 107.1 (C-8), 103.0 (OCH2O), 97.1 (C-1'''), 72.1 (C-5'''), 71.7, 71.6, 71.5, 71.2 (C-3'''), 69.6 (C-2'''), 68.6 (C-4'''), 68.6 (C-11), 65.1 (C-6''), 63.8 (C-2), 62.9 (C-6'''), 56.8 (3', 5'-OCH3), 46.7 (C-4), 45.1 (C-1), 39.9 (C-3), 36.8 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 36.7 (COCH2), 19.4 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 19.3 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3), 14.0 (CH2CH3); ESIMS: m/z 1210 [M+Na]+, HRESIMS: calcd for C58H81N3O23H [M+H]+ 1188.5334, found 1188.5298.
4β-[4-(α-d-Glucopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxy-4'-demethylpodophyllotoxin (42). White amorphous powder, yield 97% (after chromatography with CHCl3/CH3OH, 9:1); mp 148 °C;1H-NMR (CD3OD, 400 MHz): δ 8.22 (s, 1H, C5''-H), 6.61 (s, 1H, C5-H), 6.59 (s, 2H, C2', C6'-H), 6.25 (s, 1H, C8-H), 6.03–5.93 (m, 3H, C4-H, OCH2O), 5.03 (d, 1H, J = 12.0 Hz, C1'''-H), 4.85 (s, 2H, C6''-CH2), 4.70 (d, 1H, J = 4.3 Hz, C1-H), 4.43 (d, 1H, J = 4.0 Hz), 4.24–4.16 (m, 2H, C6'''-CH2), 3.91 (d, 1H, J = 12.0 Hz), 3.79 (s, 6H, C3'', C5''-OCH3), 3.68 (dd, 1H, J = 4.0 Hz, 12.0 Hz, C2-H), 3.57–3.46 (m, 1H, C3-H), 3.39–3.35 (m, 2H), 3.27–3.21 (m, 2H); 13C-NMR (CD3OD, 100 MHz): δ 176.1 (C-12), 149.7 (C-7), 149.2 (C-6), 148.6 (C-3', C-5'), 145.9 (C-4''), 135.6 (C-1'), 134.2 (C-9), 131.6 (C-10), 129.2 (C-4'), 125.8 (C-5''), 110.9 (C-5), 109.2 (C-2', C-6'), 107.2 (C-8), 103.8 (C-1'''), 103.1 (OCH2O), 78.1 (C-5'''), 77.9 (C-3'''), 75.0 (C-2'''), 71.6 (C-4'''), 71.3 (C-11), 63.9 (C-2), 63.2 (C-6''), 62.7 (C-6'''), 56.8 (3', 5'-OCH3), 46.6 (C-4), 45.1 (C-1), 39.9 (C-3); ESIMS: m/z 666 [M+Na]+, HRESIMS: calcd for C30H33N3O13Na [M+Na]+ 666.1906, found 666.1900.
4β-{4-[1-(β-d-Glucopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (43). White amorphous powder, yield 92% (after chromatography with CHCl3/CH3OH, 9:1); mp 104–106 °C; [α]D25.1: −40.3 (c 0.18, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.82 (s, 1H, C5''-H), 6.68 (s, 1H, C5-H), 6.64 (s, 1H, C8-H), 6.38 (s, 2H, C2', C6'-H), 6.25 (d, 2H, J = 4.8 Hz, C4-H), 5.97 (d, 2H, J = 5.0 Hz, OCH2O), 4.78 (d, 1H, J = 4.0 Hz, C1-H), 4.62 (s, 2H, C6''-CH2), 4.31 (d, 1H, J = 8.0 Hz, C1'''-H), 4.02–3.98 (m, 2H), 3.87–3.79 (m, 2H), 3.74 (s, 6H, C3'', C5''-OCH3), 3.70–3.61 (m, 12H), 3.43–3.39 (m, 2H), 3.28–3.26 (m, 2H), 3.21–3.13 (m, 2H); 13C-NMR (CD3OD, 100 MHz): δ 176.0 (C-12), 150.5 (C-7), 149.2 (C-6) , 148.7 (C-3', C-5'), 145.9 (C-4''), 135.1 (C-9), 131.3 (C-10), 131.3 (C-1'), 126.9 (C-4'), 125.9 (C-5''), 111.2 (C-5), 109.7 (C-8), 109.3 (C-2', C-6'), 104.4 (OCH2O), 103.2 (C-1'''), 77.9 (C-5'''), 77.9 (C-3'''), 75.1 (C-2'''), 71.6 (C-4'''), 71.4, 71.4, 70.9 (C-11), 69.6, 68.9, 64.9 (C-6''), 62.7 (C-6'''), 59.8 (C-2), 56.7 (3', 5'-OCH3), 44.7 (C-4), 42.7 (C-1), 38.5 (C-3); ESIMS: m/z 798 [M+Na]+, HRESIMS: calcd for C36H45N3O13Na [M+Na]+ 798.2692, found 798.2669.
4β-{4-[1-(β-d-Glucopyranosyloxymethyl)-3,6,9,12,15,18-hexaoxanonadec-19-yl]-1,2,3-triazol-1-yl}-4-deoxy-4'-demethylpodophyllotoxin (44). White amorphous powder, yield 94% (after chromatography with CHCl3/CH3OH, 9:1); [α]D24.9: −37.7 (c 0.23, CH3OH); 1H-NMR (CD3OD, 400 MHz): δ 7.81 (s, 1H, C5''-H), 6.67 (s, 1H, C5-H), 6.63 (s, 1H, C8-H), 6.38 (s, 2H, C2', C6'-H), 6.24 (d, 1H, J = 4.7 Hz, C4-H), 5.97 (d, 2H, J = 5.3 Hz, OCH2O), 4.77 (d, 1H, J = 4.0 Hz, C1-H), 4.64 (s, 2H, C6''-CH2), 4.35 (d, 1H, J = 8.0 Hz, C1'''-H), 4.04–4.00 (m, 2H), 3.88–3.78 (m, 2H), 3.75 (s, 6H, C3'', C5''-OCH3), 3.68–3.63 (m, 24H), 3.48–3.39 (m, 2H), 3.31–3.29 (m, 2H), 3.25–3.21 (m, 1H), 3.17–3.11 (m, 1H); 13C-NMR (CD3OD, 100 MHz,): δ 176.0 (C-12), 150.5 (C-7), 149.2 (C-6), 148.7 (C-3', C-5'), 146.0 (C-4''), 136.2 (C-1'), 135.1 (C-9), 131.3 (C-10), 126.9 (C-4'), 125.9 (C-5''), 111.2 (C-5), 109.7 (C-8), 109.3 (C-2', C-6'), 104.4 (OCH2O), 103.2 (C-1'''), 77.9 (C-5'''), 77.9 (C-3'''), 75.1 (C-2'''), 71.6 (C-4'''), 71.1, 71.0, 70.9, 70.9 (C-11), 69.6, 68.9, 65.0 (C-6''), 62.7 (C-6'''), 59.8 (C-2), 56.8 (3', 5'-OCH3), 44.7 (C-4), 42.7 (C-1), 38.5 (C-3); ESIMS: m/z 931 [M+Na]+, HRESIMS: calcd for C42H57N3O19Na [M+Na]+ 930.3478, found 930.3465.