Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene
Abstract
:1. Introduction
2. Results and Discussion
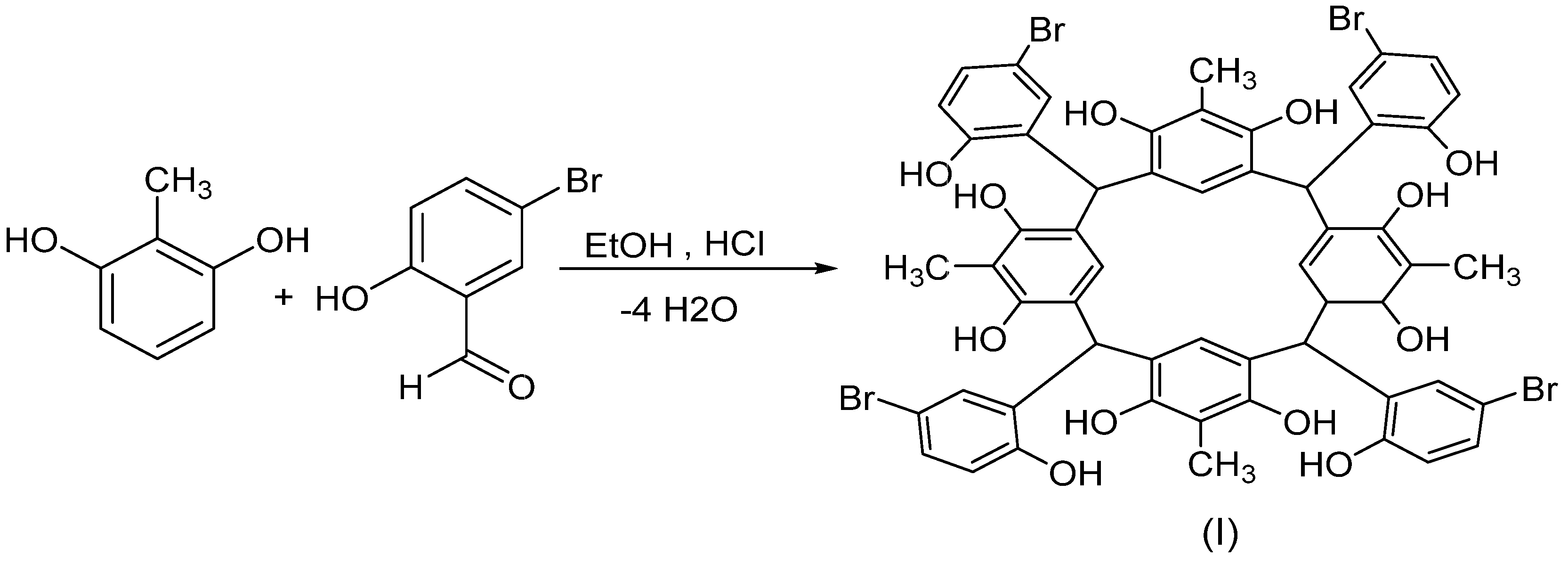
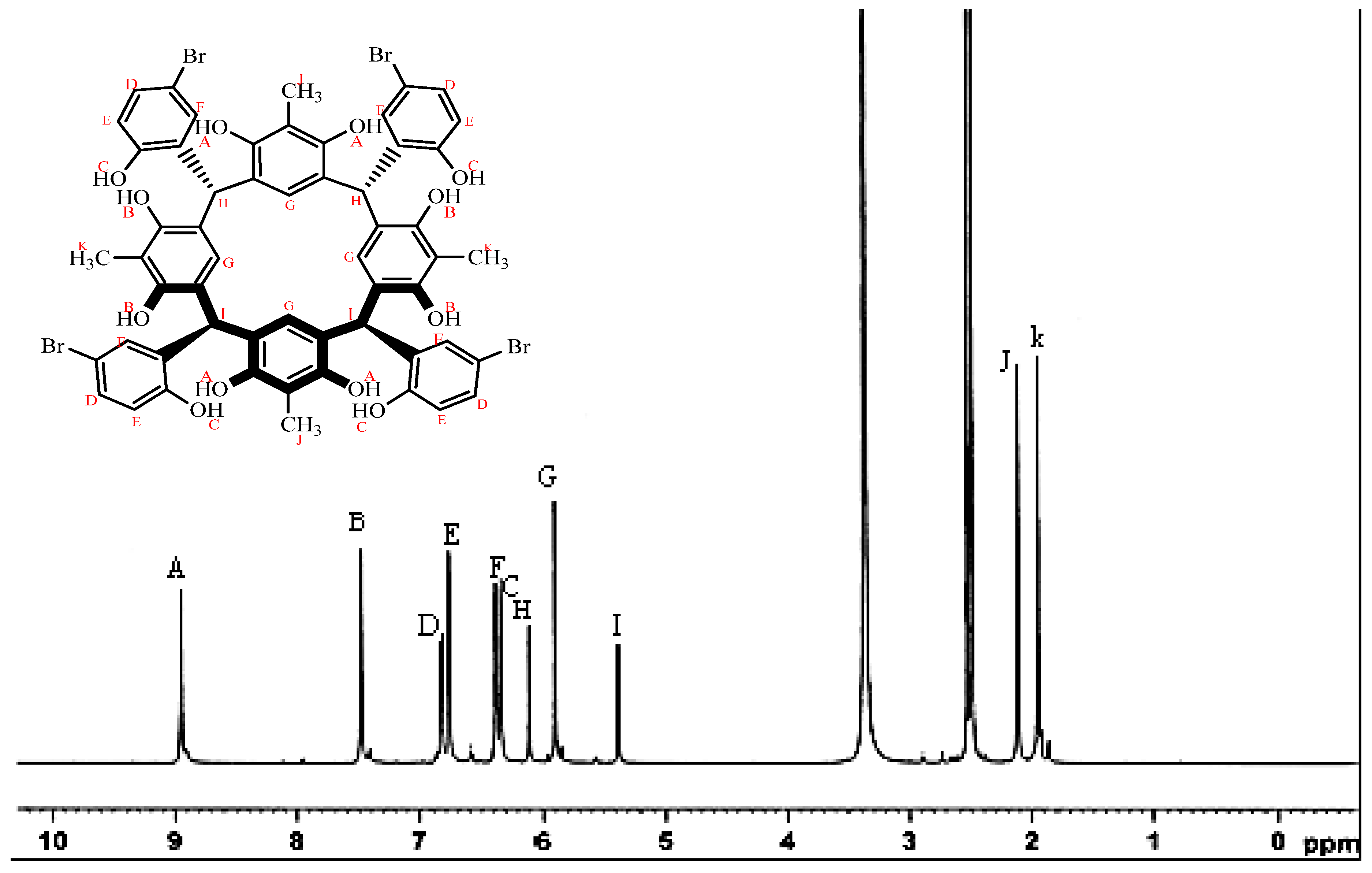
2.1. Synthesis and Characterization
2.2. X-Ray Structure

| Bond | Length Å | Bond | Angles ° |
|---|---|---|---|
| Br1-C13 | 1.905(6) | C12-C13-Br1 | 120.0(4) |
| Br2-C27 | 1.902(5) | C26-C27-Br2 | 120.6(4) |
| Br3-C41 | 1.904(6) | C40-C41-Br3 | 118.8(4) |
| Br4-C55 | 1.905(5) | C54-C55-Br4 | 119.8(4) |
| O1-C3 | 1.381(6) | O1-C3-C2 | 116.9(5) |
| O2-C5 | 1.390(6) | O2-C5-C4 | 117.2(5) |
| O3-C10 | 1.359(7) | O3-C10-C11 | 122.6(5) |
| O4-C16 | 1.366(6) | O4-C16-C15 | 116.0(5) |
| N1-C59 | 1.319(8) | C59-N1-C58 | 120.6(6) |
| N2-C62 | 1.314(9) | C62-N2-C60 | 120.6(6) |
| N3-C65 | 1.325(8) | C65-N3-C64 | 121.5(5) |
| N4-C68 | 1.327(8) | C68-N4-C67 | 120.8(6) |

| D―H….A | D―H | H….A | H….A | D―H….A |
|---|---|---|---|---|
| O1W―H1WB.....O17 | 0.82(6) | 1.94(6) | 2.756(8) | 172(3) |
| O3―H3….O20 | 0.84 | 1.86 | 2.690(6) | 169 |
| O2W―H2WB…..O20 | 0.82(4) | 2.08(4) | 2.895(7) | 173(6) |
| O7―H7….O15 | 0.84 | 1.83 | 2.576(6) | 146 |
| O12―H12….O2W | 0.84 | 1.86 | 2.696(7) | 174 |
| C64―H64B….O1W | 0.98 | 2.60 | 3.514(9) | 155 |
| C66―H66B….O3W | 0.98 | 2.59 | 3.400(9) | 140 |
| C72―H72B….O4 | 0.98 | 2.36 | 3.337(9) | 172 |
| C76―H76A….O18 | 0.98 | 2.59 | 3.440(8) | 145 |
| O1―H1…..O19 ii | 0.84 | 2.08 | 2.821(6) | 148 |
| O1W―H1WA….O13 vii | 0.82(7) | 2.03(7) | 2.815(7) | 161(6) |
| O2W―H2WA….O16 ix | 0.82(3) | 1.91(4) | 2.718(7) | 168(10) |
| O4―H4….O3W iii | 0.84 | 1.9 | 2.668(6) | 152 |
| O5―H5….O7 iv | 0.84 | 2.06 | 2.796(5) | 145 |
| O3W―H3WA….O13 xi | 0.82(5) | 1.96(6) | 2.756(7) | 162(6) |
| O6―H6….O1W iv | 0.84 | 1.89 | 2.718(7) | 170 |
| O3W―H3WB....O14 x | 0.82(5) | 2.13(5) | 2.945(7) | 174(9) |
| O4W―H4WA….O17 viii | 0.82(6) | 1.94(6) | 2.764(8) | 174(7) |
| O8―H8….O18 v | 0.84 | 2.01 | 2.811(6) | 159 |
| O4W―H4WB....O14 iii | 0.82(5) | 1.98(5) | 2.778(10) | 166(7) |
| O9―H9….O4W vi | 0.84 | 1.87 | 2.706(7) | 170 |
| O10―H10….O18 v | 0.84 | 1.91 | 2.730(6) | 165 |
| O11―H11….O1 i | 0.84 | 2.05 | 2.786(5) | 147 |
| C21―H21A….O3W iii | 0.98 | 2.37 | 3.325(9) | 165 |
| C21―H21B….O7 iv | 0.98 | 2.47 | 3.305(7) | 143 |
| C21―H21C….O6 iv | 0.98 | 2.53 | 3.455(8) | 158 |
| C36―H36….O18 v | 0.98 | 2.38 | 3.255(7) | 146 |
| C49―H49B….O1 i | 0.98 | 2.59 | 3.444(6) | 146 |
| C59―H59….O4W vi | 0.95 | 2.51 | 3.441(8) | 167 |
| C61―H61B….O5 iv | 0.98 | 2.57 | 3.509(7) | 160 |
| C75―H75A….O15 iii | 0.98 | 2.53 | 3.453(8) | 157 |
| C78―H78A….O16 ix | 0.98 | 2.59 | 3.331(9) | 133 |
| C79―H79C….O3 ii | 0.98 | 2.55 | 3.487(8) | 161 |
| C80―H80….O16 iii | 0.95 | 2.59 | 3.531(8) | 171 |
2.3. Thermogravimetric Study

2.4. Biological Studies
2.4.1. Antioxidant Properties
2.4.2. Antibacterial Activity

| Dose (µg) | Diameter of inhibition zone (mm) | ||||
|---|---|---|---|---|---|
| MRSA | Sa | Ef | Ea | Pa | |
| 250 | 13 | 13 | 15 | 6 | 6 |
| 125 | 12 | 12 | 13 | 6 | 6 |
| 62.5 | 12 | 11 | 11 | 6 | 6 |
| 31.25 | 11 | 11 | 11 | 6 | 6 |
| 15.63 | 10 | 10 | 10 | 6 | 6 |
| Antibiotic control (30 µg) | 15 a | 22 b | 23 b | 26 b | 16 b |
| DMSO (solvent control) | 6 | 6 | 6 | 6 | 6 |
| Microorganism | MIC mg/mL | MBC mg/mL | SI |
|---|---|---|---|
| MRSA (Gram-positive) | 1.563 | 25 | 0.256 |
| Sa (Gram-positive) | 6.25 | 12.5 | 0.064 |
| Ef (Gram-positive) | 6.25 | 12.5 | 0.064 |
| Ea (Gram-negative) | >25 | - | - |
| Pa (Gram-negative) | >25 | - | - |
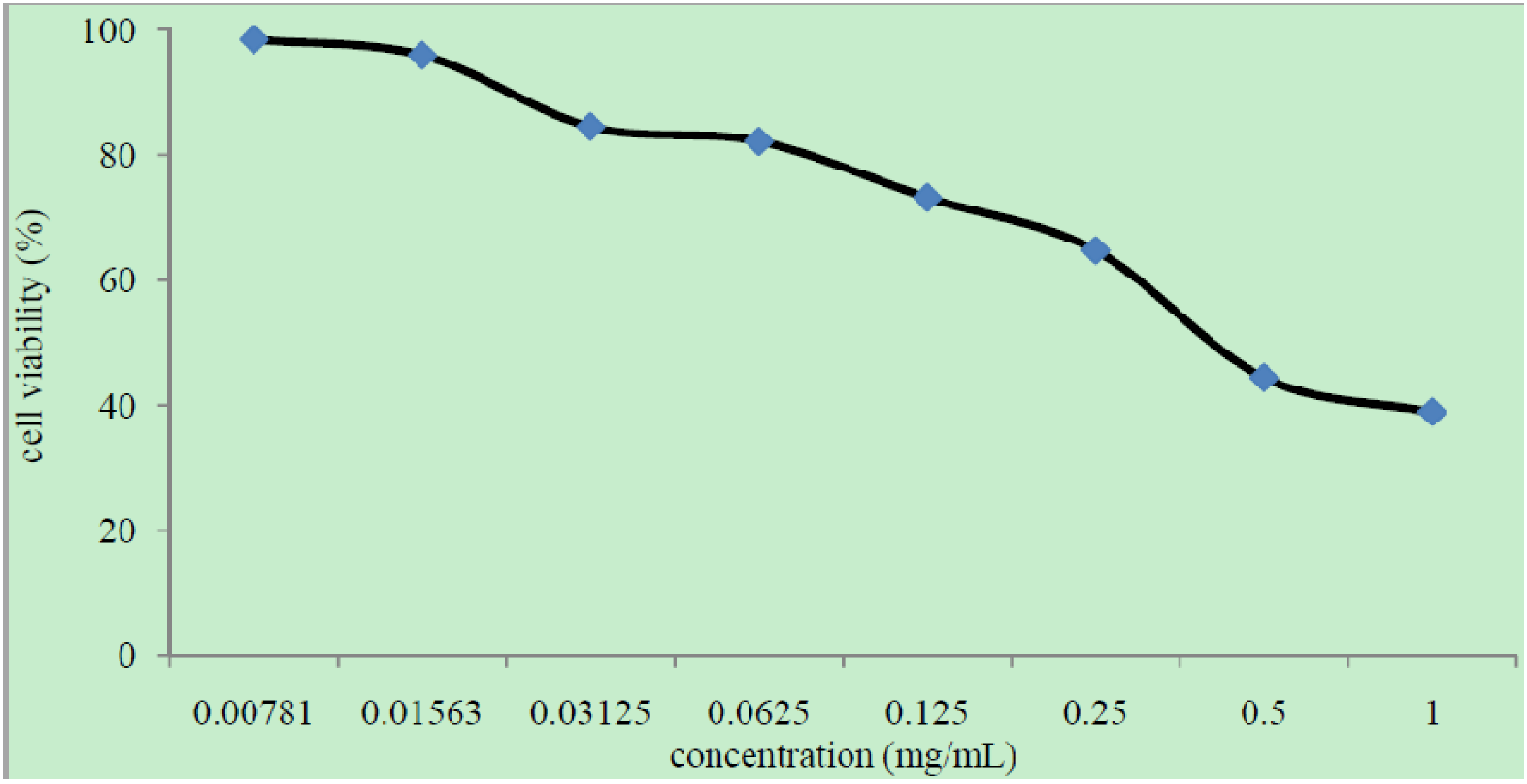
2.4.3. Cytotoxicity Studies
3. Experimental
3.1. Materials and Physical Measurements
3.2. Preparation of C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene (I)
3.3. X-ray Crystallography
| Crystal parameters | Data/values | |
|---|---|---|
| CCDC deposition number | 959177 | |
| Empirical formula | C80H108Br4N8O24 | |
| Moiety formula | C56H44Br4O12, 8(C3H7NO), 4(H2O) | |
| Formula weight | 1885.38 | |
| Temperature | 100(2) K | |
| Wavelength λ | 0.71073 Å | |
| Crystal system | Triclinic | |
| Space group | Pī | |
| Unit cell dimensions | α = 68.656(3)° | |
| β = 85.689(3)° | ||
| γ = 81.631(3)° | ||
| Volume | 4258.6(7) Å3 | |
| Z | 2 | |
| Dcal (Mg/m3) | 1.470 | |
| Absorption coefficient | 1.969 mm−1 | |
| F(000) | 1952 | |
| Crystal dimension (mm) | 0.42 × 0.37 × 0.24 | |
| Tmin/Tmax | 0.4918, 0.6494 | |
| Reflections measured | 130536 | |
| Ranges/indices (h,k,l) | −19, 19; −20, 20; −21, 21 | |
| θ limits (º) | 2.8 to 26.0° | |
| Unique reflections | 16701 | |
| Observed reflections (I>2σ(I)) | 11455 | |
| Parameters | 1101 | |
| Goodness of fit on F2 | 1.13 | |
| R1, wR2 (I≥2σ(I)) | 0.0661, 0.1652 | |
| R1,wR2 indices (all data) | 0.1107, 0.1997 | |
| Largest diff. peak and hole | 2.669 and −0.977 e.Å−3 | |
3.4. Antioxidant Test
3.5. Antibacterial Activity
3.6. Cytotoxicity Evaluation
4. Conclusions
Supplementary Material
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Formation of linear supramolecular polymers that is driven by C–H⋅⋅⋅π interactions in solution and in the solid state. Angew. Chem. Int. 2011, 50, 1397–1401. [Google Scholar] [CrossRef]
- Han, C.; Ma, F.; Zhang, Z.; Xia, B.; Huang, F. DIBPillar[n]arenes (n = 5, 6): Syntheses, X-ray crystal structures, and complexation with n-Octyltriethyl ammonium hexafluorophosphate. Org. Lett. 2010, 12, 4360–4363. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-healing supramolecular gels formed by crown ether based host—Guest interactions. Angew. Chem. Int. 2012, 51, 7011–7015. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Turshatov, A.A.; Melnikova, N.B.; Semchikov, Y.D.; Ryzhkina, I.S.; Pashirova, T.N.; Mobius, D.; Zaitsev, S.Y. Interaction of monolayers of calix[4]resorcinarene derivatives with copper ions in the aqueous subphase. Colloids Surf. A Physicochem. Eng. Aspects. 2004, 240, 101–106. [Google Scholar] [CrossRef]
- Sarkar, A.; Krishnamurthy, S.S.; Nethaji, M. Calix[4]arene bisphosphite ligands bearing two distal 2,2-biphenyldioxy conformational flexibility and allyl-palladium complexes. Tetrahedron. 2009, 65, 374–382. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Burmakina, N.E.; Syakaev, V.V.; Sudakova, N.S.; Shagidullin, R.R.; Konovalov, A.I. Synthesis IR and NMR characterization and ion extraction properties of tetranonylcalix[4] resorcinol bearing acetylhydrazone groups. Tetrahedron. 2009, 65, 408–417. [Google Scholar] [CrossRef]
- Hedidi, M.; Hamdi, S.M.; Mazari, T.; Boutemeur, B.; Rabia, C.; Chemat, F.; Hamdi, M. Microwave-assisted synthesis of calix[4]resorsinarene. Tetrahedron. 2006, 62, 5652–5655. [Google Scholar] [CrossRef]
- Ruderisch, A.; Pfeiffer, J.; Schurig, V. Synthesis of an enantiomerically pure resorcinarene with pendant L-valine residues and its attachment to a polysiloxane(Chirasil-Calix). Tetrahedron Asymmetry. 2001, 12, 2025–2030. [Google Scholar] [CrossRef]
- Makinen, M.; Jalkanen, J.P.; Vainiotalo, P. Conformational properties and intramolecular hydrogen bonding of tetraethyl resorcinarene: An ab initio study. Tetrahedron. 2002, 58, 8591–8596. [Google Scholar] [CrossRef]
- Thondorf, I.; Brenn, J.; Bohme, V. Conformational properties of methylene-bridged resorcarenes. Tetrahedron. 1998, 54, 12823–12828. [Google Scholar] [CrossRef]
- Iwanek, W.; Wzorek, A. Introduction to the chirality of resorcinarenes. Mini Rev. Org. Chem. 2009, 6, 398–411. [Google Scholar] [CrossRef]
- Davis, C.J.; Lewis, P.T.; Billodeaux, D.R.; Fronczek, F.R.; Escobedo, J.O.; Strongin, R.M. Solid-state supramolecular structures of resorcinol-arylboronic acid compounds. Org. Lett. 2001, 3, 2443–2445. [Google Scholar] [CrossRef]
- Knyanzeva, I.R.; Burilov, A.R.; Fazleeva, G.M.; Nuretdinov, I.A.; Gryanznova, T.V.; Budnikova, Y.G.; Khrisanforova, V.V.; Gubaidullin, A.T.; Gabidullin, B.M.; Syakaey, V.V.; et al. New Calix[4]resorcinols with Thiophosphoryl-containing fragments. Phosphorus Sulfur Silicon. 2011, 186, 1972–1980. [Google Scholar] [CrossRef]
- Aishahateet, S.F.; Kooli, F.; Messali, M.; Judeh, Z.M.A.; Eldouhaibi, A.S. Synthesis and supramolecularity of C-phenylcalix[4]pyrogallolarenes: Temperature effect on the formation of different isomers. Mol. Cryst. Liq. Cryst. 2007, 474, 89–110. [Google Scholar] [CrossRef]
- Patel, M.B.; Modi, N.R.; Raval, J.P.; Menon, S.K. Calix[4]arene based 1,3,4-oxadiazole and thiadiazole derivatives: Design, synthesis and biological evaluation. Org. Biomol. Chem. 2012, 10, 1785–179. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Al-Adiwish, W.M.; Tahir, M.I.M.; Siti-Noor-Adnalizawati, A.; Hashim, S.F.; Ibrahim, N.; Yaacob, W.A. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo [1,5-a] pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Zirihi, G.N.; Mambu, L.; Guede-Guina, F.; Bodo, B.; Grellier, P. In vitro anti-plasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005, 98, 281–285. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for crystal structure determination. In SHELXTL; Version 6.14; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C. Antibiotic susceptibility testing by a standard single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Andrighetti-Frohner, C.R; Antonio, R.; Creczynski-Pasa, T.; Barardi, C.; Simoes, C. Citotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem. Inst. Oswaldo Cruz. 2003, 98, 843–848. [Google Scholar] [CrossRef]
- Jordao, A.K.; Ferreira, V.F.; Thiago, M.L.S.; Gabrielle, C.S.F.; Viviane, M.; Juliana, L.A.; Maria, C.B.V.; Anna, C.C. Synthesis and anti-HSV-1 activity of new 1,2,3-triazole derivatives. Biorg. Med. Chem. 2011, 19, 1860–865. [Google Scholar] [CrossRef]
- Macedo, N.R.P.V.; Ribeiro, M.S.; Villaca, R.C.; Ferreira, W.; Pinto, A.M.; Teixeira, V.L.; Cirne-Santos, C.; Paixao, I.C.N.P.; Giongo, V. Caulerpin as a potential antiviral drug against herpes simplex virus type 1. Braz. J. Pharmacogn. 2012, 22, 861–867. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abosadiya, H.M.; Hasbullah, S.A.; Mackeen, M.M.; Low, S.C.; Ibrahim, N.; Koketsu, M.; Yamin, B.M. Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene. Molecules 2013, 18, 13369-13384. https://doi.org/10.3390/molecules181113369
Abosadiya HM, Hasbullah SA, Mackeen MM, Low SC, Ibrahim N, Koketsu M, Yamin BM. Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene. Molecules. 2013; 18(11):13369-13384. https://doi.org/10.3390/molecules181113369
Chicago/Turabian StyleAbosadiya, Hamza M., Siti Aishah Hasbullah, Mukram Mohamed Mackeen, Seow Chew Low, Nazlina Ibrahim, Mamoru Koketsu, and Bohari M. Yamin. 2013. "Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene" Molecules 18, no. 11: 13369-13384. https://doi.org/10.3390/molecules181113369
APA StyleAbosadiya, H. M., Hasbullah, S. A., Mackeen, M. M., Low, S. C., Ibrahim, N., Koketsu, M., & Yamin, B. M. (2013). Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene. Molecules, 18(11), 13369-13384. https://doi.org/10.3390/molecules181113369