A Commonly Used Chinese Herbal Formula, Shu-Jing-Hwo-Shiee-Tang, Potentiates Anticoagulant Activity of Warfarin in a Rabbit Model
Abstract
:1. Introduction
2. Results
2.1. Warfarin, but not SJHST, Induced Coagulopathy When Used Alone
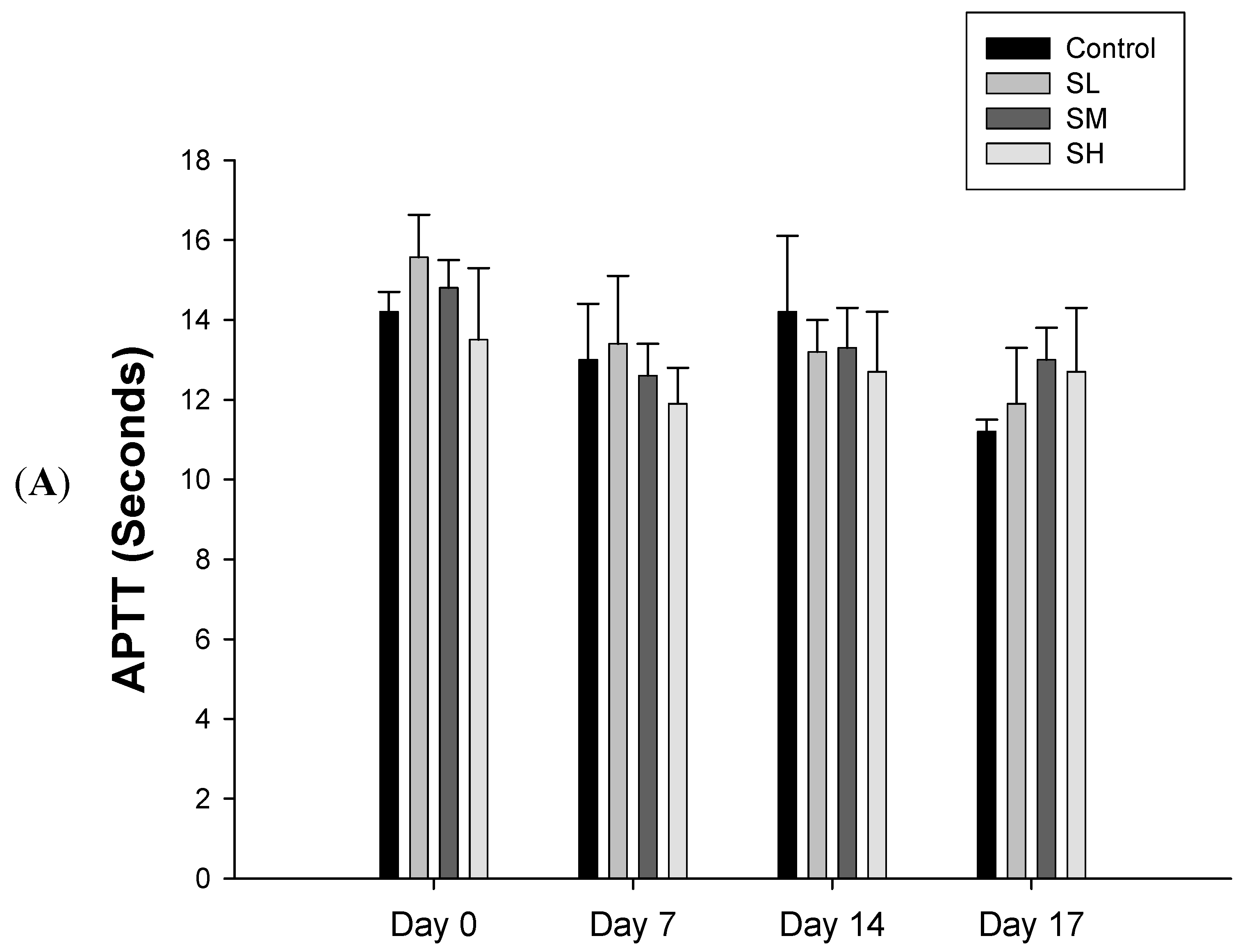
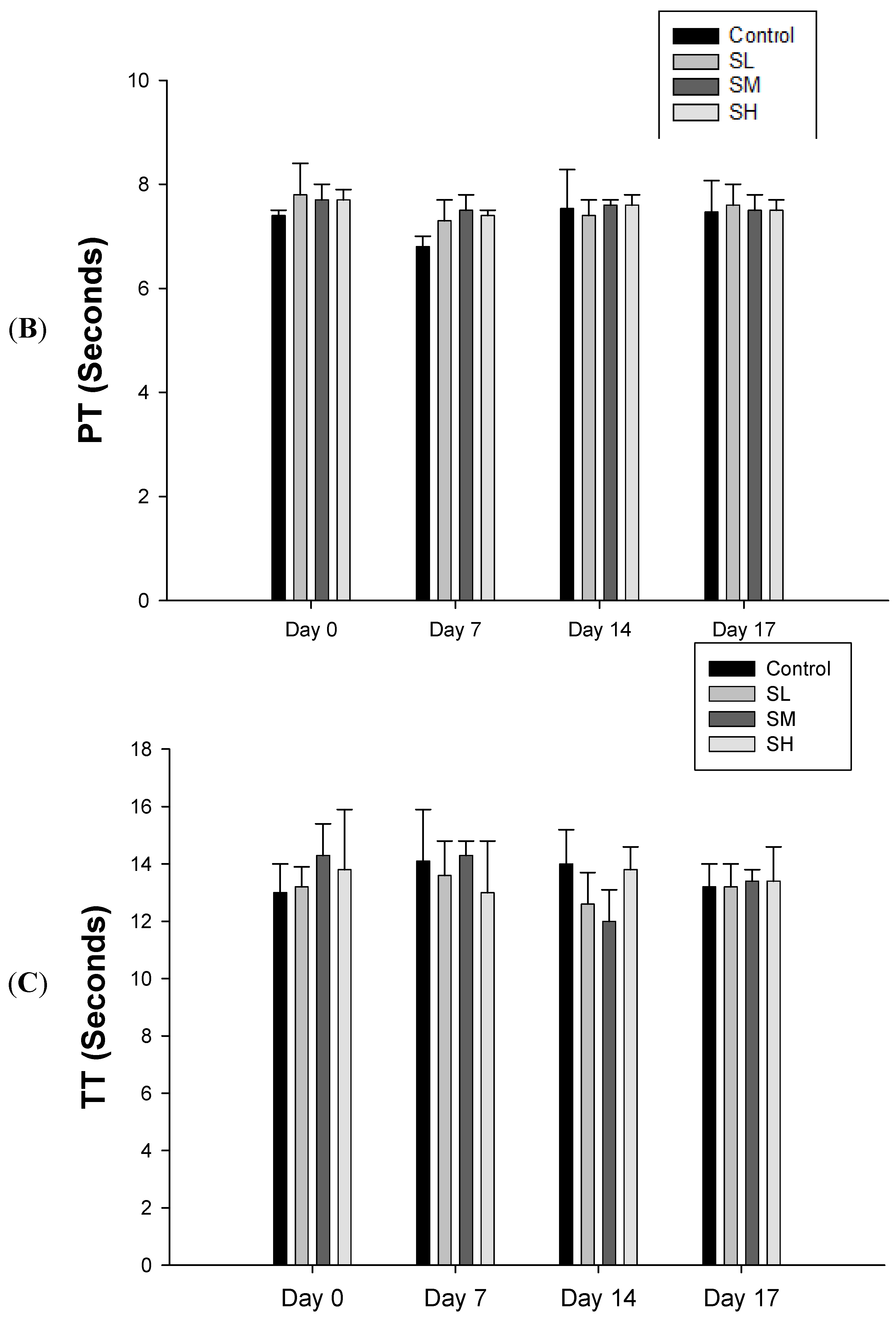
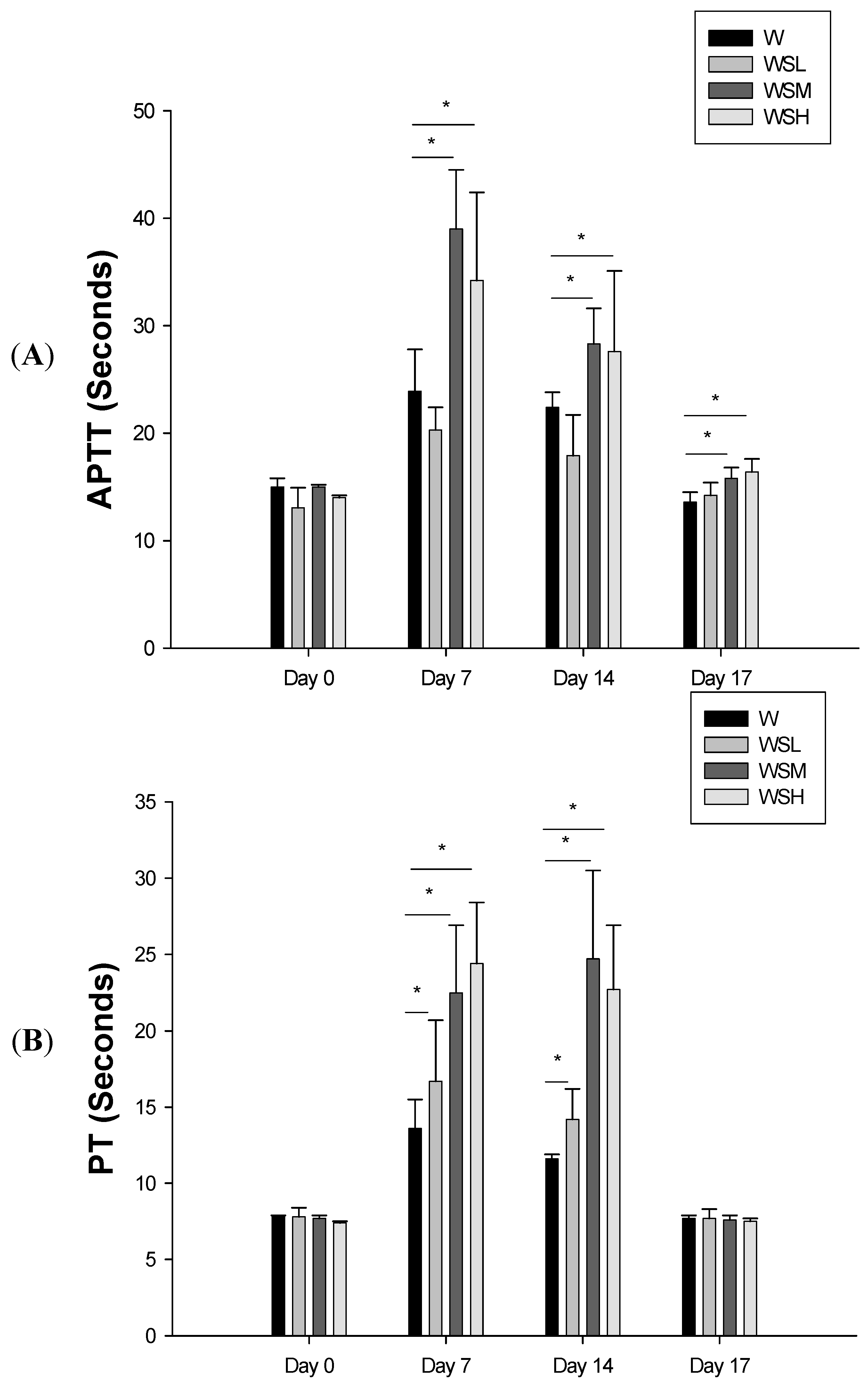

2.2. Concurrent Use of Warfarin and SJHST may Induce Coagulopathy
3. Discussion
4. Experimental
4.1. Animals
4.2. Medications
| Latin name | Plant part | Ratio of composition |
|---|---|---|
| Angelica sinensis (Oliv.) Diels. | root | 2 |
| Ligusticum chuanxiong Hort. | Stem and root | 1 |
| Paeonia lactiflora Pall. | root | 2.5 |
| Atractylodes lancea (Thunb.) DC. or Atractylodes chinensis | rhizome | 2 |
| Poria cocos (Schw.) Wolf | Fungus | 1 |
| Glycyrrhiza uralensis Fisch. | Root | 1 |
| Rehmannia glutinosa (Gaertn.) DC. | Root | 2 |
| Clematis chinensis Osbeck | Root | 2 |
| Zingiber officinale Rosc | Root | 3 |
| Prunus persica (L.) Batsch | seed | 2 |
| Cyathula officinalis Kuan | root | 2 |
| Stephania tetrandra S. Moore | root | 1 |
| Gentiana manshurica Kitag, Gentiana scabra Bge., Gentiana triflora Pall., or Gentiana regescens Franch. | root | 1 |
| Angelica dahurica Benth. et Hood. F or Angelica dahurica Benth. et Hook. F. var. formosana Shan et Yuan | root | 1 |
| Citrus reticulate Blanco | fruit | 2 |
| Notopterygium incisum Ting. ex H. T. Chang | Stem and root | 1 |
| Saposhnikovia divariata (Turcz.) Schischk. | Root | 1 |
4.3. Procedures
4.4. Measurement of Coagulation Profile
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Almadi, M.A.; Barkun, A.; Brophy, J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: An 86-year-old woman with peptic ulcer disease. JAMA 2011, 306, 2367–2374. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lin, H.W.; Lu, Y.H.; Chen, Y.L.; Mahady, G.B. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One 2013, 8, e64255. [Google Scholar]
- Nutescu, E.A.; Shapiro, N.L.; Ibrahim, S.; West, P. Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin. Drug Saf. 2006, 5, 433–451. [Google Scholar] [CrossRef]
- Samuels, N. Herbal remedies and anticoagulant therapy. Thromb. Haemost. 2005, 93, 3–7. [Google Scholar]
- Lo, A.C.; Chan, K.; Yeung, J.H.; Woo, K.S. Danggui (Angelica sinensis) affects the pharmacodynamics but not the pharmacokinetics of warfarin in rabbits. Eur. J. Drug Metab. Pharmacokinet. 1995, 20, 55–60. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, G.; Chen, B.; Xie, Y.; Wu, H.; Wu, Y.; Yan, C.; Wang, J. Separation and quantitative analysis of coumarin compounds from Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook. f by pressurized capillary electrochromatography. J. Pharm. Biomed. Anal. 2006, 41, 105–116. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, J.; Zhang, S.; Zhou, H.H.; Toma, D.; Ren, S.; Huang, L.; Yaramus, M.; Baum, A.; Venkataramanan, R.; Xie, W. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J. Pharmacol. Exp. Ther. 2006, 316, 1369–1377. [Google Scholar]
- Shalansky, S.; Lynd, L.; Richardson, K.; Ingaszewski, A.; Kerr, C. Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: A longitudinal analysis. Pharmacotherapy 2007, 27, 1237–1247. [Google Scholar] [CrossRef]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health Syst. Pharm. 2000, 57, 1221–1227. [Google Scholar]
- Page, R.L., 2nd; Lawrence, J.D. Potentiation of warfarin by dong quai. Pharmacotherapy 1999, 19, 870–876. [Google Scholar] [CrossRef]
- Lesho, E.P.; Saullo, L.; Udvari-Nagy, S. A 76-year-old woman with erratic anticoagulation. Cleve. Clin. J. Med. 2004, 71, 651–656. [Google Scholar] [CrossRef]
- Liapina, L.A.; Ammosova Ia, M.; Novikov, V.S.; Osipova, N.N.; Smolina, T.; Pastorova, V.E.; Uspenskaia, M.S.; Liapin, G. The nature of an anticoagulant isolated from peonies in the central zone of Russia. Izv. Akad. Nauk. Ser. Biol. 1997, 1997, 235–237. [Google Scholar]
- Wu, L.C.; Lin, X.; Sun, H. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC). Acta Pharmacol. Sin. 2012, 33, 1254–1259. [Google Scholar] [CrossRef]
- Chen, X.W.; Sneed, K.B.; Pan, S.Y.; Cao, C.; Kanwar, J.R.; Chew, H.; Zhou, S.F. Herb-drug interactions and mechanistic and clinical considerations. Curr. Drug Metab. 2012, 13, 640–651. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Drug interactions with herbal medicines. Clin. Pharmacokinet. 2012, 51, 77–104. [Google Scholar] [CrossRef]
- Verhoef, T.I.; Redekop, W.K.; Daly, A.K.; van Schie, R.M.; de Boer, A.; Maitland-van der Zee, A.H. Pharmacogenetic-guided dosing of coumarin anticoagulants: Algorithms for warfarin, acenocoumarol and phenprocoumon. Br. J. Clin. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Zhan, C.; Yang, J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 2006, 53, 303–309. [Google Scholar] [CrossRef]
- Tawata, M.; Aida, K.; Noguchi, T.; Ozaki, Y.; Kume, S.; Sasaki, H.; Chin, M.; Onaya, T. Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur. J. Pharmacol. 1992, 212, 87–92. [Google Scholar] [CrossRef]
- Jin, Y.R.; Han, X.H.; Zhang, Y.H.; Lee, J.J.; Lim, Y.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-gamma2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research CDER. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf (accessed on 16 September 2013).
Appendix

- Sample Availability: Samples of the compounds contained in SJHST are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, S.-H.; Yu, C.-L.; Chen, H.-Y.; Lin, Y.-H. A Commonly Used Chinese Herbal Formula, Shu-Jing-Hwo-Shiee-Tang, Potentiates Anticoagulant Activity of Warfarin in a Rabbit Model. Molecules 2013, 18, 11712-11723. https://doi.org/10.3390/molecules181011712
Yang S-H, Yu C-L, Chen H-Y, Lin Y-H. A Commonly Used Chinese Herbal Formula, Shu-Jing-Hwo-Shiee-Tang, Potentiates Anticoagulant Activity of Warfarin in a Rabbit Model. Molecules. 2013; 18(10):11712-11723. https://doi.org/10.3390/molecules181011712
Chicago/Turabian StyleYang, Sien-Hung, Chia-Li Yu, Hsing-Yu Chen, and Yi-Hsuan Lin. 2013. "A Commonly Used Chinese Herbal Formula, Shu-Jing-Hwo-Shiee-Tang, Potentiates Anticoagulant Activity of Warfarin in a Rabbit Model" Molecules 18, no. 10: 11712-11723. https://doi.org/10.3390/molecules181011712





