Enaminonitriles in Heterocyclic Synthesis: A Route to 1,3-Diaryl-4-aminopyrazole Derivatives
Abstract
:1. Introduction

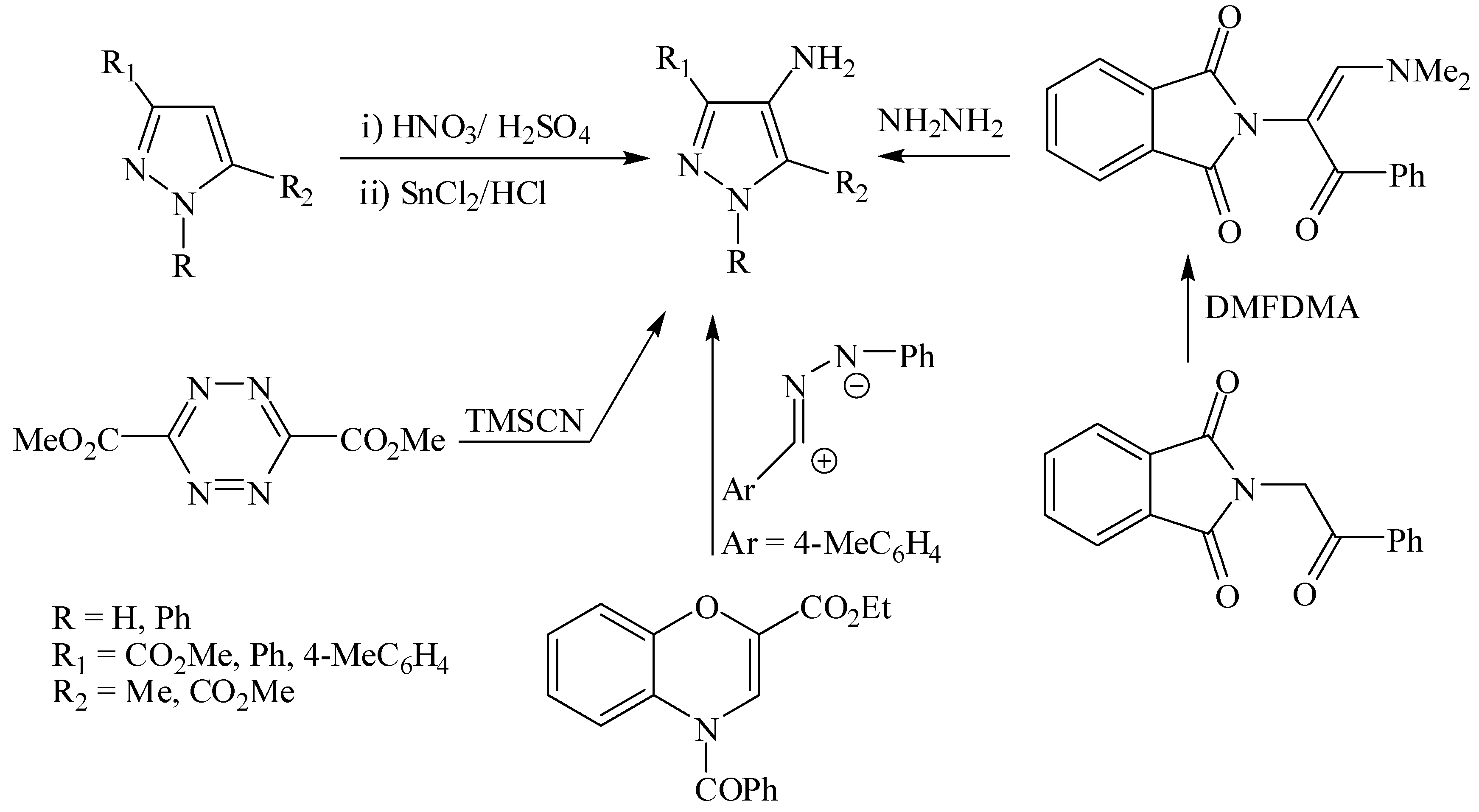
2. Results and Discussion
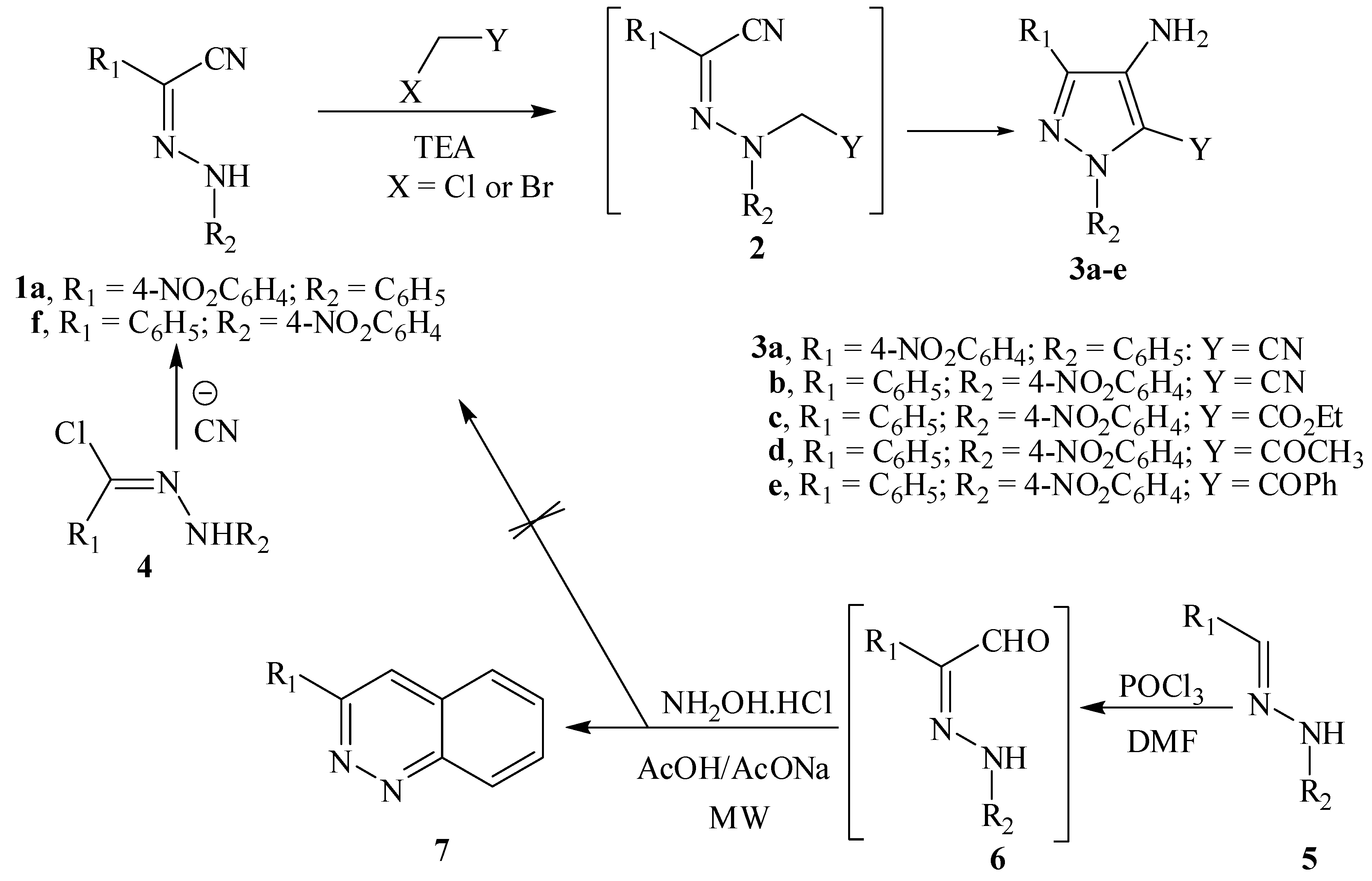
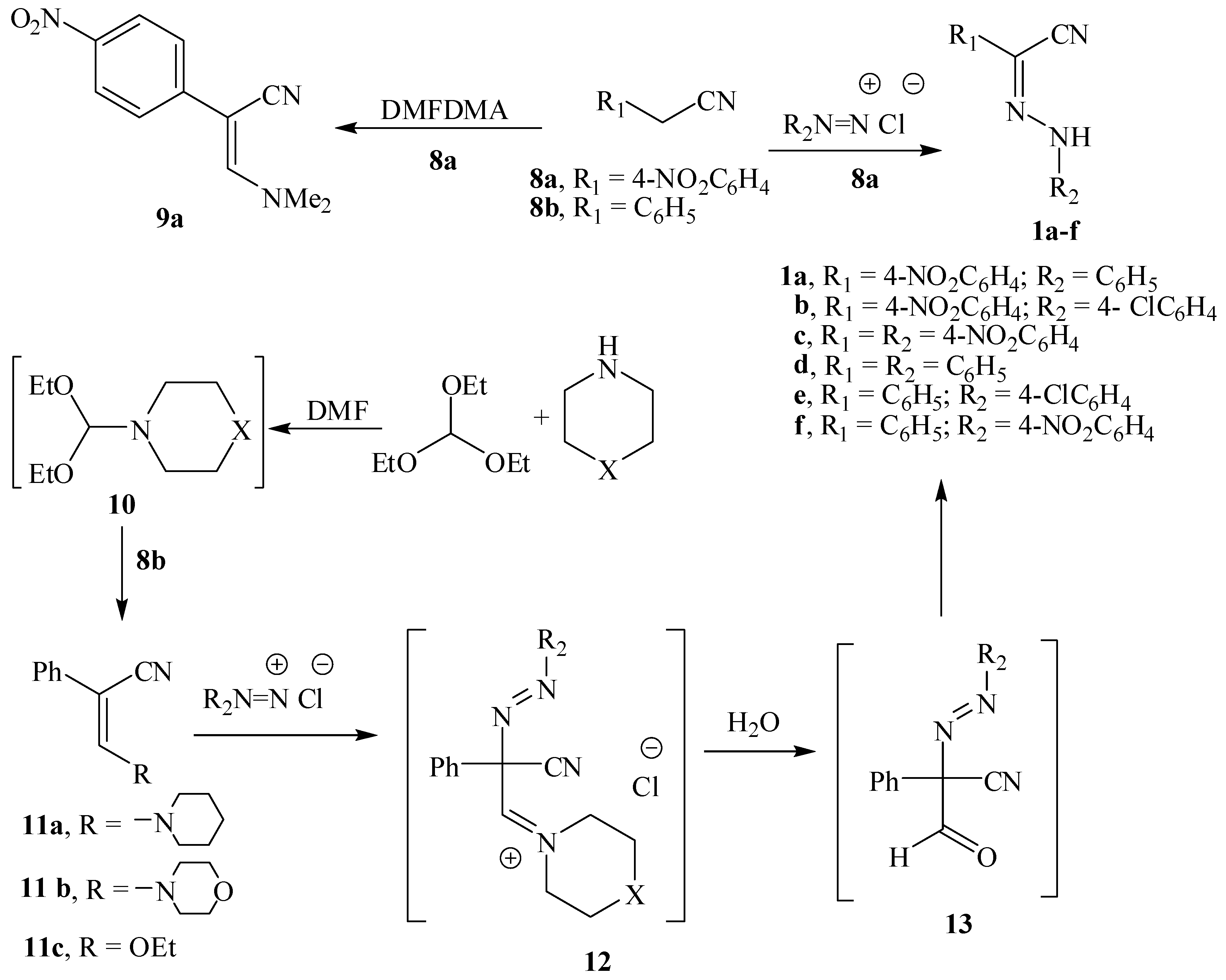
3. Experimental
3.1. General
3.2. Preparation of Substituted Aryl Hydrazonoacetonitriles 1a–f
3.3. General Procedure for Preparation of 4-Aminopyrazole Derivatives 3a–e
3.4. 3-Dimethylamino-2-(4-nitrophenyl) acrylonitrile (9a)
3.5. 3-Substituted Amino-2-phenylacrylonitriles 11a,b
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 1a–f, 9a and 11a,b are available from the authors.
References
- Baxendale, I.R.; Ley, S.V. Polymer-supported reagents for multi-step organic synthesis: Application to the synthesis of sildenafil. Bioorg. Med. Chem. Lett. 2000, 10, 1983–1986. [Google Scholar] [CrossRef]
- El-Abdelah, M.M.; Sabri, S.S.; Khanfar, M.A.; Yassin, H.A.; Volter, W. Synthesis and properties of isoviagra. A 2-methyl-2H-pyrazolo[4,3-d] pyrirnidin-7-one isomer of Viagra. J. Heterocycl. Chem. 2002, 39, 1055–1059. [Google Scholar] [CrossRef]
- Pace, A.; Buscemi, S.; Vivona, N.; Caronna, T. Sensitized Photoreduction of Nitrosoazoles on Titanium Dioxide. Heterocycles 2000, 53, 183–190. [Google Scholar] [CrossRef]
- Tahir, M.; Goreg, R.H.; Brain, P.; Nicola, C.N. Convenient synthesis of 4-amino-3,5-disubstituted pyrazoles in one step from the corresponding diketo oximes. Tetrahedron Lett. 2004, 45, 2137–2139. [Google Scholar]
- Boolell, M.; Gepi-Attee, S.; Gingell, J.C.; Allen, M.J. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br. J. Urol. 1996, 78, 257–261. [Google Scholar]
- Moreland, R.B.; Goldstein, I.; Traish, A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998, 62, 309–318. [Google Scholar]
- Ravi, P.; Tewari, S.P. Facile and environmentally friendly synthesis of nitropyrazoles using montmorillonite K-10 impregnated with bismuth nitrate. Catal. Commun. 2012, 19, 37–41. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, H. Ring transformation reaction of 1,2,4,5-tetrazines to 4-aminopyrazoles by cyanotrimethylsilane. Tetrahedron Lett. 1998, 28, 2139–2142. [Google Scholar] [CrossRef]
- Anwar, H.F.; Elnagdi, M.H. Recent developments in aminopyrazole chemistry. Arkivoc 2009, i, 198–250. [Google Scholar]
- Fagan, P.J.; Neidert, E.E.; Nye, M.J.; O’Hare, M.J.; Tang, W.P. Cycloadditions and other chemistry of 4-oxygenated pyrazoles. Can. J. Chem. 1979, 57, 904–912. [Google Scholar] [CrossRef]
- Chen, C.; Wilcoxen, K.; McCarthy, J.R. A convenient one-pot synthesis of 4-amino-3-arylpyrazoles from α-phthaloylaminoacetophenones. Tetrahedron Lett. 1998, 39, 8229–8232. [Google Scholar]
- Carpenter, R.D.; Lam, K.S.; Kurth, M.J.; Richard, D.; Carpenter, K.S.L.; Mark, J.K. Microwave-Mediated Heterocyclization to Benzimidazo[2,1-b]quinazolin-12(5H)-ones. J. Org. Chem. 2007, 72, 284–287. [Google Scholar]
- Caddick, S. Microwave assisted organic reactions. Tetrahedron 1995, 51, 10403–10432. [Google Scholar] [CrossRef]
- Majetich, G.; Wheless, K. Microwave-Enhanced Chemistry; Kinsington, H.M., Haswell, S.J., Eds.; American Chemical Society: Washington, DC, USA, 1997; p. 455. [Google Scholar]
- Salaheldin, A.M.; Al-Sheikh, M.A. β-Enamino Esters in Heterocyclic Synthesis: Synthesis of Pyrazolone and Pyridinone Derivatives. Molecules 2010, 15, 4359–4368. [Google Scholar] [CrossRef]
- Salaheldin, A.M.; Abdallah, T.A.; Radwan, N.F.; Hassaneen, H.M. A Novel Route to 4-Aminopyrazoles and Aminopyrazolo[4,3-b]pyridines. Z. Naturforsch. 2006, 61b, 1158–1161. [Google Scholar]
- Abdallah, T.A.; Salaheldin, A.M.; Radwan, N.F. Studies with enamines: Synthesis and reactivity of 4-nitrophenyl-1-piperidinostyrene. Synthesis of 4-nitrophenyl-1-piperidinostyrene. Synthesis of pyridazine, oxadiazole, 1,2,3-triazole and 4-aminopyrazole derivatives. Z. Naturforsch. 2007, 62b, 261–266. [Google Scholar]
- Al-Shiekh, M.A.; Medrassi, H.Y.; Elnagdi, M.H.; Hafez, E.A. Substituted hydrazonals as building blocks in heterocyclic synthesis: A new route to arylhydrazonocinnolines. J. Chem. Res. 2007, 432–436. [Google Scholar]
- Almazroa, S.; Salaheldin, A.M.; Elnagdi, M.H. Studies with enaminones: The reaction of enaminones with aminoheterocycles. A route to azolopyrimidines, azolopyridines and quinolines. J. Heterocycl. Chem. 2004, 41, 267–272. [Google Scholar] [CrossRef]
- Al-Shiekh, M.A.; Salaheldin, A.M.; Hafez, E.A.; Elnagdi, M.H. 2-arylhydrazono-3-oxopropanals in heterocyclic synthesis: synthesis of arylazopyrazole, arylazoisooxazole and dialkylpyridazine-5,6-dicarboxylate. New one step synthesis of arylazopyrimidine. J. Heterocycl. Chem. 2004, 41, 647–654. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelkader, M.H.; Eltalbawy, F.M.A. Synthesis and tautomeric structure of novel 3,7-bis(arylazo)-2,6-diphenyl-1H-imidazo-[1,2-b]pyrazoles in ground and excited states. Tetrahedron 2002, 58, 2875–2878. [Google Scholar] [CrossRef]
- Salaheldin, A.M.; Alphy, M.A. Studies with Enaminonitriles: Synthesis and Chemical Reactivity of 2-Phenyl-3-Piperidin-1-yl Acrylonitrile under Microwave Heating. J. Heterocycl. Chem. 2008, 45, 307–310. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Riyadh, S.M.; Elnagdi, M.H. 2-Arylhydrazononitriles in heterocyclic synthesis: A novel route to 1,3-diaryl-1,2,4-triazol-5-amines via a Tiemann rearrangement of arylhydrazonoamidoximes. Arkivoc 2007, xiii, 53–62. [Google Scholar]
- Laue, T.; Plagens, A. Named Organic Reactions; John Wiley & Sons: New York, NY, USA, 1998; p. 161. [Google Scholar]
- Gewald, K.; Schäfer, H.; Bellmann, P.; Hain, U. On the synthesis of. 3-aminopyrroles by Thorpe–Ziegler cyclization. J. Prakt. Chem. 1992, 334, 491–496. [Google Scholar] [CrossRef]
- Rehwald, M.; Schäfer, H.; Gewald, K. New syntheses of 2,4-diaminopyrroles and aminopyrrolinones. Monatsh. Chem. 1997, 128, 933–943. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Medrasi, H.Y.; Al-Sheikh, M.A.; Salaheldin, A.M. Enaminonitriles in Heterocyclic Synthesis: A Route to 1,3-Diaryl-4-aminopyrazole Derivatives. Molecules 2013, 18, 535-544. https://doi.org/10.3390/molecules18010535
Medrasi HY, Al-Sheikh MA, Salaheldin AM. Enaminonitriles in Heterocyclic Synthesis: A Route to 1,3-Diaryl-4-aminopyrazole Derivatives. Molecules. 2013; 18(1):535-544. https://doi.org/10.3390/molecules18010535
Chicago/Turabian StyleMedrasi, Hanadi Y., Mariam Abdullah Al-Sheikh, and Abdellatif Mohamed Salaheldin. 2013. "Enaminonitriles in Heterocyclic Synthesis: A Route to 1,3-Diaryl-4-aminopyrazole Derivatives" Molecules 18, no. 1: 535-544. https://doi.org/10.3390/molecules18010535
APA StyleMedrasi, H. Y., Al-Sheikh, M. A., & Salaheldin, A. M. (2013). Enaminonitriles in Heterocyclic Synthesis: A Route to 1,3-Diaryl-4-aminopyrazole Derivatives. Molecules, 18(1), 535-544. https://doi.org/10.3390/molecules18010535



