Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison on Phenolic Compounds
| Analytical Index | Cabernet Sauvignon | Merlot | ||||||
|---|---|---|---|---|---|---|---|---|
| NXYQY | SXXN | HBCL | HBSC | NXYQY | SXXN | HBCL | HBSC | |
| TP a (GAE) | 2710.4 ± 200.5a | 1129.8 ± 79.6c | 1313.0 ± 45.2c | 2330.2 ± 120.3b | 1656.5 ± 161.7a | 860.2 ± 45.7c | 941.2 ± 66.4c | 1247.7 ± 110.1b |
| TFO b (CTE) | 2290.3 ± 157.2a | 859.9 ± 55.5d | 1189.1 ± 89.1c | 1906.4 ± 132.9b | 1375.4 ± 76.4a | 697.5 ± 23.1c | 660.6 ± 18.5c | 1031.4 ± 95.4b |
| TFA b (CTE) | 532.3 ± 79.8b | 277.7 ± 34.3c | 342.7 ± 47.6c | 666.4 ± 84.6a | 497.4 ± 16.4a | 279.8 ± 24.1c | 272.8 ± 40.0c | 361.1 ± 10.3b |
| TA c | 400.3 ± 16.3a | 286.8 ± 27.9b | 261.5 ± 42.5b | 372.6 ± 4.2a | 350.3 ± 54.8a | 259.4 ± 0.8b | 157.5 ± 17.5c | 216.7 ± 20.8bc |
2.1.1. Total Phenols
2.1.2. Total Flavonoids
2.1.3. Total Flavanols
2.1.4. Total Anthocyanins
2.2. Comparison on Antioxidant Activity
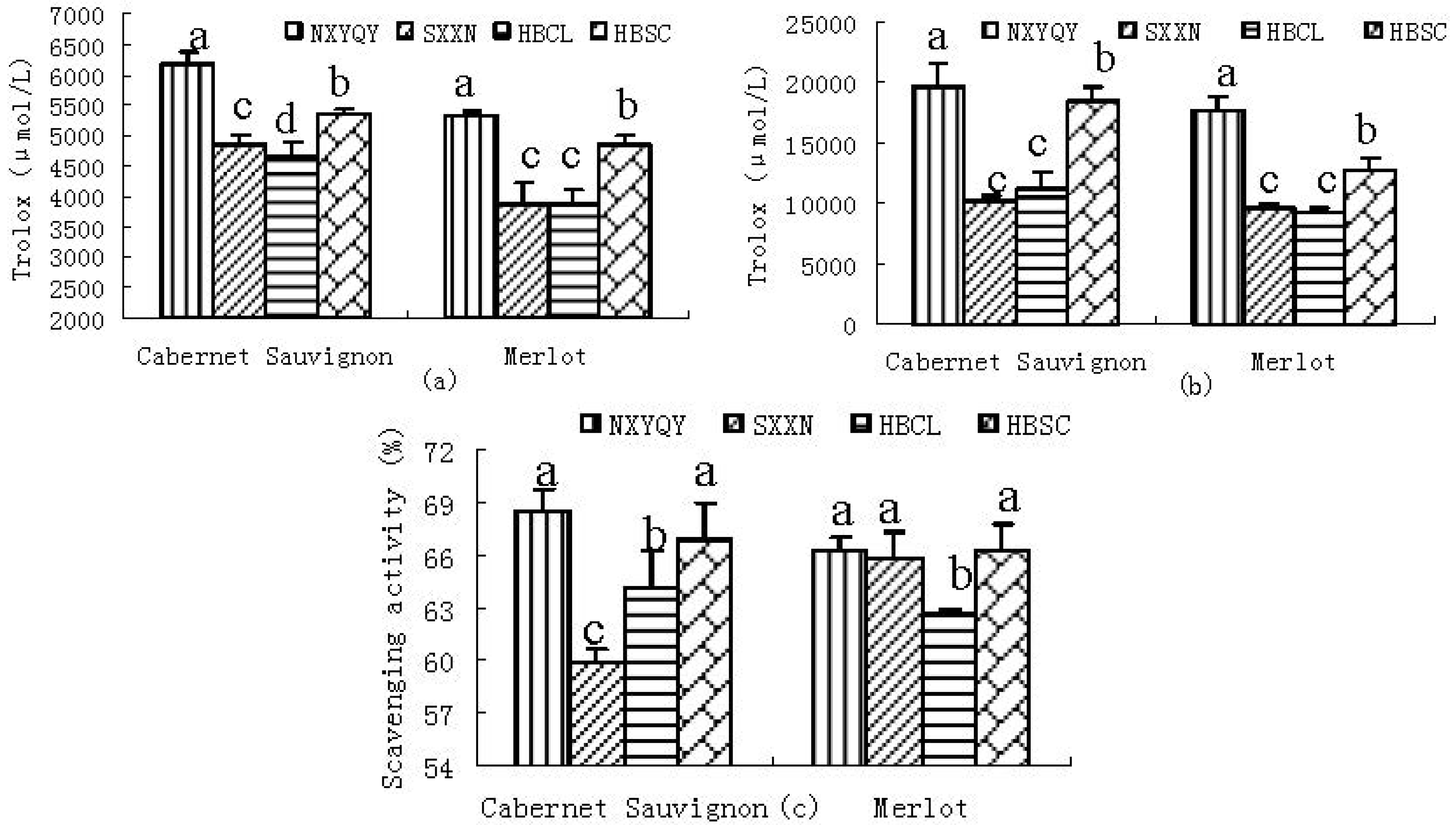
2.2.1. DPPH• Scavenging Activity
2.2.2. CUPRAC
2.2.3. SRSA
2.3. Comparison on Individual Phenolics
2.3.1. Comparison on Flavan-3-ols
| Compound | Cabernet Sauvignon Wine | Merlot Wine | Retention Time (Min) | Molecular Ions (M+) | Fragment Ions (MS2, m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NXYQY | SXXN | HBSC | HBCL | NXYQY | SXXN | HBSC | HBCL | ||||
| Flavan-3-ols | |||||||||||
| Catechin | 62.4 ± 3.7 | 8.1 ± 0.8 | 48.2 ± 9.6 | 45.2 ± 3.5 | 23.2 ± 3.5 | 15.4 ± 2.1 | 29.5 ± 2.0 | 14.9 ± 4.8 | 3.14 | 289 | 245, 205 |
| Epicatechin | 81.8 ± 11.7 | 6.0 ± 0.1 | 40.5 ± 4.7 | 31.5 ± 2.3 | 32.2 ± 0.7 | 16.8 ± 2.4 | 42.7 ± 5.9 | 27.1 ± 2.8 | 6.12 | 289 | 245 |
| Gallocatechin | 1.9 ± 0.1 | — | — | — | — | — | 1.0 ± 0.1 | — | 0.36 | 305 | 179, 125 |
| Procyanidin dimer B2 | 54.7 ± 5.8 | 14.0 ± 1.3 | 14.9 ± 1.7 | 7.6 ± 0.6 | 22.7 ± 4.4 | 7.8 ± 1.4 | 5.7 ± 0.4 | 25.1 ± 1.3 | 2.32 | 577 | 425, 289 |
| Procyanidin dimer B3 | 55.7 ± 4.1 | 1.6 ± 0.6 | 7.6 ± 0.8 | 9.4 ± 0.8 | 5.3 ± 0.6 | 2.2 ± 0.3 | 7.8 ± 0.4 | 23.7 ± 1.4 | 3.27 | 865 | 695, 577, 287 |
| Flavonols | |||||||||||
| Myricetin-3-O-galactoside | 2.2 ± 0.1 | 3.0 ± 0.2 | 5.6 ± 0.6 | 1.6 ± 0.1 | — | 4.8 ± 0.4 | 0.9 ± 0.0 | 3.9 ± 0.4 | 13.34 | 479 | 317 |
| Myricetin-3-O-glucoside | 4.0 ± 0.6 | 2.2 ± 0.3 | — | 4.1 ± 0.8 | 5.0 ± 0.7 | 6.0 ± 0.3 | 6.8 ± 0.1 | 5.5 ± 0.3 | 14.12 | 479 | 317, 179 |
| Myricetin-3-O-glucuronide | — | — | 1.4 ± 0.1 | — | — | — | trace | — | 13.57 | 493 | 317 |
| Quercetin-3-O-hexoside | 2.9 ± 0.4 | 1.5 ± 0.3 | — | — | — | — | 9.0 ± 0.7 | — | 4.43 | 463 | 301 |
| Quercetin-3-O-galactoside | 6.4 ± 0.4 | 5.5 ± 0.3 | — | 3.1 ± 0.0 | 7.1 ± 1.4 | 5.1 ± 0.6 | 6.5 ± 0.5 | — | 19.15 | 463 | 301 |
| Quercetin-3-O-glucuronide | 11.0 ± 0.3 | 1.4 ± 0.1 | 4.6 ± 0.4 | 4.8 ± 0.3 | 11.9 ± 0.6 | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.4 | 19.68 | 477 | 301 |
| Quercetin-3-O-glucoside | — | 2.1 ± 0.1 | — | 3.8 ± 0.4 | — | 2.6 ± 0.2 | 5.3 ± 0.8 | — | 20.84 | 463 | 301 |
| Dihydroquercetin-3-O-rhamnoside | 10.5 ± 0.7 | 2.7 ± 0.3 | 5.4 ± 0.4 | 6.6 ± 0.6 | 8.9 ± 0.6 | 8.1 ± 0.3 | 1.1 ± 0.0 | 7.1 ± 0.3 | 18.84 | 449 | 285, 151 |
| Laricitrin-3-O-glucoside | 13.7 ± 0.6 | 0.9 ± 0.1 | 5.0 ± 0.7 | 6.0 ± 0.4 | 8.2 ± 0.4 | 1.9 ± 0.1 | 5.0 ± 0.3 | 5.5 ± 0.6 | 23.13 | 493 | 331 |
| Kaempferol-3-O-galactoside | 5.2 ± 0.3 | 1.5 ± 0.1 | — | 2.3 ± 0.0 | — | 2.3 ± 0.1 | 5.1 ± 0.4 | 3.5 ± 0.3 | 23.57 | 447 | 285 |
| Kaempferol-3-O-glucoside | — | 2.3 ± 0.3 | — | 4.3 ± 0.4 | 4.8 ± 0.4 | 2.7 ± 0.2 | — | — | 24.08 | 447 | 285 |
| Isorhamnetin-3-O-glucoside | 9.6 ± 0.3 | 1.6 ± 0.1 | — | 2.8 ± 0.3 | 4.5 ± 0.6 | 2.3 ± 0.0 | 2.1 ± 0.1 | — | 29.66 | 477 | 314 |
| Isorhamnetin-3-O-galactoside | — | 3.0 ± 0.4 | — | — | — | — | 4.3 ± 0.6 | — | 7.45 | 477 | 315 |
| Hydroxybenzoic acids | |||||||||||
| DEGCEC a | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.0 | trace | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.1 ± 0.0 | 1.4 ± 0.3 | 0.36 | 593 | 425, 289, 407 |
| Hx-es-va b | — | — | — | — | — | — | 2.1 ± 0.1 | — | 4.94 | 329 | 167, 191 |
| Syringic acid | 5.2 ± 0.4 | 0.8 ± 0.2 | 1.5 ± 0.1 | 3.8 ± 0.4 | 5.0 ± 0.6 | 0.3 ± 0.0 | 0.9 ± 0.1 | 11.3 ± 1.1 | 8.07 | 197 | 182, 153 |
| Hx-es-pc-a c | 1.9 ± 0.4 | — | — | 0.8 ± 0.0 | — | — | 4.7 ± 0.5 | — | 1.81 | 315 | 153 |
| Hydroxycinnamic acids | |||||||||||
| Cinnamic acid | 1.7 ± 0.3 | 0.9 ± 0.2 | — | 0.9 ± 0.1 | 1.4 ± 0.0 | 0.8 ± 0.1 | — | 1.2 ± 0.2 | 38.90 | 147 | |
| trans-Fertaric acid | — | 0.9 ± 0.0 | 1.6 ± 0.1 | 0.9 ± 0.2 | 4.3 ± 0.3 | 1.1 ± 0.2 | — | 0.7 ± 0.1 | 3.04 | 325 | 193 |
| Hx-es-fa d | — | — | — | — | — | — | 0.9 ± 0.1 | — | 1.27 | 355 | 193 |
| Stilbenes | |||||||||||
| trans-Piceid | — | 0.5 ± 0.0 | 11.6 ± 0.8 | — | 1.5 ± 0.2 | 4.3 ± 0.4 | 7.5 ± 0.8 | 1.8 ± 0.3 | 24.83 | 389 | 227 |
2.3.2. Comparison of Flavonols
2.3.3. Comparison on Phenolic Acids
2.3.4. Comparison on Stilbene
2.4. Correlation
| TP | TFO | TFA | TA | DPPH | CUPRAC | SRSA | |
|---|---|---|---|---|---|---|---|
| TP | 1 | 0.962 ** | 0.967 ** | 0.856 ** | 0.875 ** | 0.945 ** | 0.641 ns |
| TFO | 1 | 0.884 ** | 0.865 ** | 0.920 ** | 0.933 ** | 0.700 ns | |
| TFA | 1 | 0.811 * | 0.771 * | 0.938 ** | 0.693 ns | ||
| TA | 1 | 0.852 ** | 0.874 ** | 0.551 ns | |||
| DPPH | 1 | 0.899 ** | 0.537 ns | ||||
| CUPRAC | 1 | 0.746 * | |||||
| SRSA | 1 |
| Phenolic Compounds | DPPH method | CUPRAC method | SRSA method | Phenolic Compounds | DPPH method | CUPRAC method | SRSA method |
|---|---|---|---|---|---|---|---|
| Catechin | 0.730 * | 0.723 * | 0.715 * | Kaempferol-3-O-galactoside | 0.539 | 0.710 | 0.752 |
| Epicatechin | 0.747 * | 0.743 * | 0.783 * | Kaempferol-3-O-glucoside | 0.585 | 0.792 | 0.613 |
| Gallocatechin | — | — | — | Isorhamnetin-3-O-glucoside | 0.845 * | 0.883 * | 0.685 |
| Procyanidin dimer B2 | 0.630 | 0.587 | 0.385 | Isorhamnetin-3-O-galactoside | — | — | — |
| Procyanidin dimer B3 | 0.529 | 0.451 | 0.435 | DEGCEC a | −0.634 | −0.585 | −0.477 |
| Myricetin-3-O-galactoside | −0.242 | 0.078 | 0.022 | Hx-es-va b | — | — | — |
| Myricetin-3-O-glucoside | −0.362 | −0.081 | 0.505 | Syringic acid | −0.120 | −0.042 | −0.100 |
| Myricetin-3-O-glucuronide | — | — | — | Hx-es-pc-a c | −0.135 | −0.076 | 0.248 |
| Quercetin-3-O-hexoside | −0.328 | −0.089 | 0.425 | Cinnamic acid | 0.772 | 0.873 * | 0.629 |
| Quercetin-3-O-galactoside | 0.491 | 0.624 | 0.385 | trans-Fertaric acid | 0.604 | 0.731 | 0.506 |
| Quercetin-3-O-glucuronide | 0.642 | 0.755 * | 0.673 | Hx-es-fa d | - | - | - |
| Quercetin-3-O-glucoside | 0.402 | 0.955 * | 0.659 | trans-Piceid | 0.356 | 0.521 | 0.683 |
| Dihydroquercetin-3-O-rhamnoside | 0.222 | 0.393 | 0.458 | Laricitrin-3-O-glucoside | 0.725 * | 0.731 * | 0.671 |
3. Experimental
3.1. Sample Collection and Vinification
| Regions | Grape Growers (Sampling Sources) | Regional soil types | Regional Climate Conditions |
|---|---|---|---|
| Yuquanying of Ningxia, (NXYQY) | Huibin Grape Co. Ltd. | Gravelly soils | The vineyards are located on the alluvial plain at the altitude of about 1,036 m with a cool and semi-arid climate and a big temperature difference between daytime and night time; with an annual accumulated temperature being 3,298–3,351 °C; with abundant sunshine and an annual rainfall of 150–200 mm. |
| Xiangning of Shanxi, (SXXN) | Chateau Rongzi Co. Ltd. | Clay loamy soil | The vineyards are located on Loess Plateau of China at the average altitude of about 1,100 m with a cold and arid climate and a big temperature difference between daytime and night time; with an annual accumulated temperature being 2,998 °C; with abundant sunshine and an annual rainfall of 400–600 mm and an annual sunshine time of 2,200–2,500 h. |
| Changli of Hebei, (HBCL) | Chateau Langes Co. Ltd. | Clay and sandy soils | The vineyards are located on the plain at the altitude of about 214 m with a cool-warm, semi-humid climate and an annual accumulated temperature of 3,940 °C; with an annual rainfall of about 700 mm and an annual sunshine time of 2,600–2,800 h. |
| Shacheng of Hebei, (HBSC) | Chateau Des Champs | Sandy soils | The vineyards are located on the Huaizhuo basin at the altitude of about 450–600 m with a warm, semi-arid climate and a big temperature difference between daytime and night time; with an annual accumulated temperature of 3,532 °C and an annual rainfall of about 413 mm. |
3.2. Reagents, Solvents and Standards
3.3. Determination of Phenolic Compounds
| Analytical Index | Cabernet Sauvignon | Merlot | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NXYQY | SXXN | HBCL | HBSC | NXYQY | SXXN | HBCL | HBSC | |||||||||
| Must | Wine | Must | Wine | Must | Wine | Must | Wine | Must | Wine | Must | Wine | Must | Wine | Must | Wine | |
| Total sugar | 217.5 ± 1.4 | — | 198.6 ± 2.0 | — | 224.1 ± 1.2 | — | 204.3 ± 2.0 | — | 215.0 ± 1.5 | — | 218.9 ± 1.6 | — | 205.3 ± 0.8 | — | 187.3 ± 0.1 | — |
| Titratable acidity (g/L) a | 8.1 ± 0.5 | 7.3 ± 0.1 | 11.9 ± 0.7 | 7.1 ± 0.3 | 7.6 ± 0.3 | 6.3 ± 0.5 | 9.3 ± 0.5 | 6.7 ± 0.2 | 7.7 ± 0.2 | 8.0 ± 0.0 | 8.3 ± 0.4 | 6.7 ± 0.4 | 8.9 ± 0.8 | 6.7 ± 0.1 | 6.9 ± 0.1 | 8.3 ± 0.3 |
| pH | 3.2 ± 0.1 | 3.1 ± 0.0 | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.5 ± 0.0 | 3.6 ± 0.1 | 3.4 ± 0.2 | 3.5 ± 0.2 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.1 | 3.6 ± 0.1 | 3.0 ± 0.1 | 3.5 ± 0.1 |
| Total tannins (g/L) b | 5.3 ± 0.3 | 2.5 ± 0.1 | 3.8 ± 0.1 | 1.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 3.5 ± 0.2 | 1.5 ± 0.0 | 2.9 ± 0.1 | 1.9 ± 0.1 | 2.6 ± 0.2 | 1.3 ± 0.1 | 2.3 ± 0.0 | 1.6 ± 0.2 | 2.1 ± 0.1 | 1.6 ± 0.1 |
| Alcohol (%) | — | 12.0 ± 0.2 | — | 11.0 ± 0.2 | — | 12.2 ± 0.1 | — | 13.4 ± 0.2 | — | 11.0 ± 0.1 | — | 10.8 ± 0.0 | — | 11.0 ± 0.1 | — | 11.5 ± 0.2 |
| Residual sugar (g/L) | — | 2.0 ± 0.1 | — | 2.1 ± 0.0 | — | 2.8 ± 0.2 | — | 2.9 ± 0.1 | — | 1.9 ± 0.0 | — | 2.1 ± 0.1 | — | 2.9 ± 0.3 | — | 3.1 ± 0.3 |
3.4. Extraction and Analyses of Individual Phenolic Compounds
3.4.1. Preparation of Sample
3.4.2. Quantitative Analyses by HPLC-MS/MS
3.4.3. Quantification of Individual Phenolic Compounds
3.5. Determination of Antioxidant Activity
3.5.1. Free Radical-Scavenging Activity on DPPH
3.5.2. Cupric Reducing Antioxidant Capacity (CUPRAC)
3.5.3. Superoxide Radical-Scavenging Activity (SRSA)
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330–335. [Google Scholar]
- Goldberg, D.M.; Hahn, S.E.; Parkes, J.G. Beyond alcohol: Beverage consumption and cardiovascular mortality. Clin. Chim. Acta 1995, 237, 155–187. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Health aspects of functional grape seed constituents. Trends Food Sci. Technol. 2004, 15, 422–433. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Miňano, A.; López-Roca, J.M. Comparison of chromatic properties, stability and antioxidant capacity of anthocyanin-based aqueous extracts from grape pomace obtained from different vinification methods. Food Chem. 2006, 97, 87–94. [Google Scholar] [CrossRef]
- Orak, H.H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlationa. Sci. Hortic. 2007, 111, 235–241. [Google Scholar]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects, Applications; Technomic Publishing Co.: Lancaster, PA, USA, 1995. [Google Scholar]
- Li, Z.; Pan, Q.H.; Jin, Z.M.; Mu, L.; Duan, C.Q. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
- Villaňo, D.; Troncoso, M.S.; Fernández-Pachón, A.M.; García-Parrilla, M.C. Influence of enological practices on the antioxidant activity of wines. Food Chem. 2006, 95, 394–404. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.Y.; Li, Y.; Li, P.H.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Tang, X.Y.; He, Z.Y.; Dai, Y.F.; Xiong, Y.L.; Xie, M.Y.; Chen, J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef]
- Zhu, L.J.; Chen, J.; Tang, X.Y.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.Y.; Zhao, M.M.; Xue, S.; Jiang, Y.M. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharm. Biomed. 2010, 51, 471–477. [Google Scholar] [CrossRef]
- Tedesco, I.; Russo, M.; Russo, P.; Iacomino, G.; Russo, G.L.; Carraturo, A.; Faruolo, C.; Molo, L.; Palumbo, R. Antioxidant effect of red wine polyphenols on red blood cells. J. Nutr. Biochem. 2000, 11, 114–119. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Flint, S.D.; Jorda, P.W.; Caldwell, M.M. Plant protective response to enchanced UV-B radiation under field conditions: Leaf optical properties of photosynthesis. Photochem. Photobiol. 1985, 41, 95–99. [Google Scholar] [CrossRef]
- Smith, G.J.; Markham, K.R. Tautomerism of flavonol glucosides: Relevance to plant UV protection and flower color. J. Photoch. Photobiol. 1998, 118, 99–105. [Google Scholar] [CrossRef]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Scheffeldt, P.; Hrazdina, G. Copigmentation of antho-cyanins under physiological conditions. J. Food Sci. 1978, 43, 517–520. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Tian, L.; Pang, Y.; Dixon, R.A. Biosynthesis and genetic engineering of proanthocyanidins and (iso) flavonoids. Phytochem. Rev. 2008, 7, 445–465. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Woraratphoka, J.; Intarapichet, K.O.; Indrapichate, K. Phenolic compounds and antioxidative properties of selected wines from the northeast of Thailand. Food Chem. 2007, 104, 1485–1490. [Google Scholar] [CrossRef]
- Price, S.F.; Breen, P.J.; Valladao, M.; Watson, B.T. Cluster sun exposure and quercetin in Pinot noir grapes and wine. Am. J. Enol. Vitic. 1995, 46, 187–194. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitisvinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Gollop, R.; Even, S.; Colova-Tsolova, V.; Perl, A. Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J. Exp. Bot. 2002, 53, 1397–1409. [Google Scholar] [CrossRef]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar]
- Cimino, F.; Sulfaro, V.; Trombetta, D.; Saija, A.; Tomaino, A. Radical-scavenging capacity of several Italian red wines. Food Chem. 2007, 103, 75–81. [Google Scholar]
- Fernández-Pachón, M.S.; Villaňo, D.; Troncoso, A.M.; García-Parrilla, M.C. Determination of the phenolic composition of sherry and table white wines by liquid chromatography and their relation with antioxidant activity. Anal. Chim. Acta 2006, 563, 101–108. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Duran, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B. A re-evaluation of the peroxynitrite scavengin activity of some dietary phenolics. Biochem. Biophys. Res. Commun. 2000, 279, 692–699. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, H.Y.; Kang, S.S.; Jung, M.J.; Kim, J.W.; Jung, H.A. The structure-activity relationship of flavonoids as scavenger of peroxynitrite. Phytother. Res. 2002, 16, 232–235. [Google Scholar] [CrossRef]
- Sadeghipour, M.; Terreux, R.; Phipps, J. Flavonoids and tyrosine nitration: Structure-Activity relationship correlation with enthalpy of formation. Toxicol. In Vitro 2005, 19, 155–165. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. Phenolic compounds and antioxidant properties of grape berries and wines in Loess Plateau region (China). Asian J. Chem. 2011, 23, 2558–2564. [Google Scholar]
- Li, H. Research Progress of Vine and Wine: College of Enology (Annual); Shaanxi Agricultural Press: Xi’an, China, 2002. [Google Scholar]
- Office International de la Vigne et du Vin. In Recueil des Methods Internationals D’analyse des vins et des mouts; Offline Image Viewer (OIV): Paris, France, 1990.
- Rapisarda, P.; Tomaino, A.; Lo Cascio, R.; Bonina, F.; de Pasquale, A.; Saija, A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- McMorrough, I.; Madigan, D.; Smyth, M.R. Semipreparative chromatographic procedure for the isolation of dimeric and trimeric proanthocyanidins from barley. J. Agric. Food Chem. 1996, 44, 1731–1735. [Google Scholar] [CrossRef]
- Giusti, M.M.; Worsltad, R.E. Current Protocols in Food Analytical Chemistry: Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Sun, J.P. Development and application of LC-UV-MS/MS library of phenolic compounds in Wines and Grapes. Ph.D. Thesis, China Agricultural University, Beijing, China. 2006. [Google Scholar]
- Brandwilliams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupricion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the wine are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, B.; Zhang, Z.-W. Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Molecules 2012, 17, 8804-8821. https://doi.org/10.3390/molecules17088804
Jiang B, Zhang Z-W. Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Molecules. 2012; 17(8):8804-8821. https://doi.org/10.3390/molecules17088804
Chicago/Turabian StyleJiang, Bao, and Zhen-Wen Zhang. 2012. "Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China" Molecules 17, no. 8: 8804-8821. https://doi.org/10.3390/molecules17088804
APA StyleJiang, B., & Zhang, Z.-W. (2012). Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Molecules, 17(8), 8804-8821. https://doi.org/10.3390/molecules17088804




