Evaluation of Antioxidant and Immunity-Enhancing Activities of Sargassum pallidum Aqueous Extract in Gastric Cancer Rats
Abstract
:1. Introduction
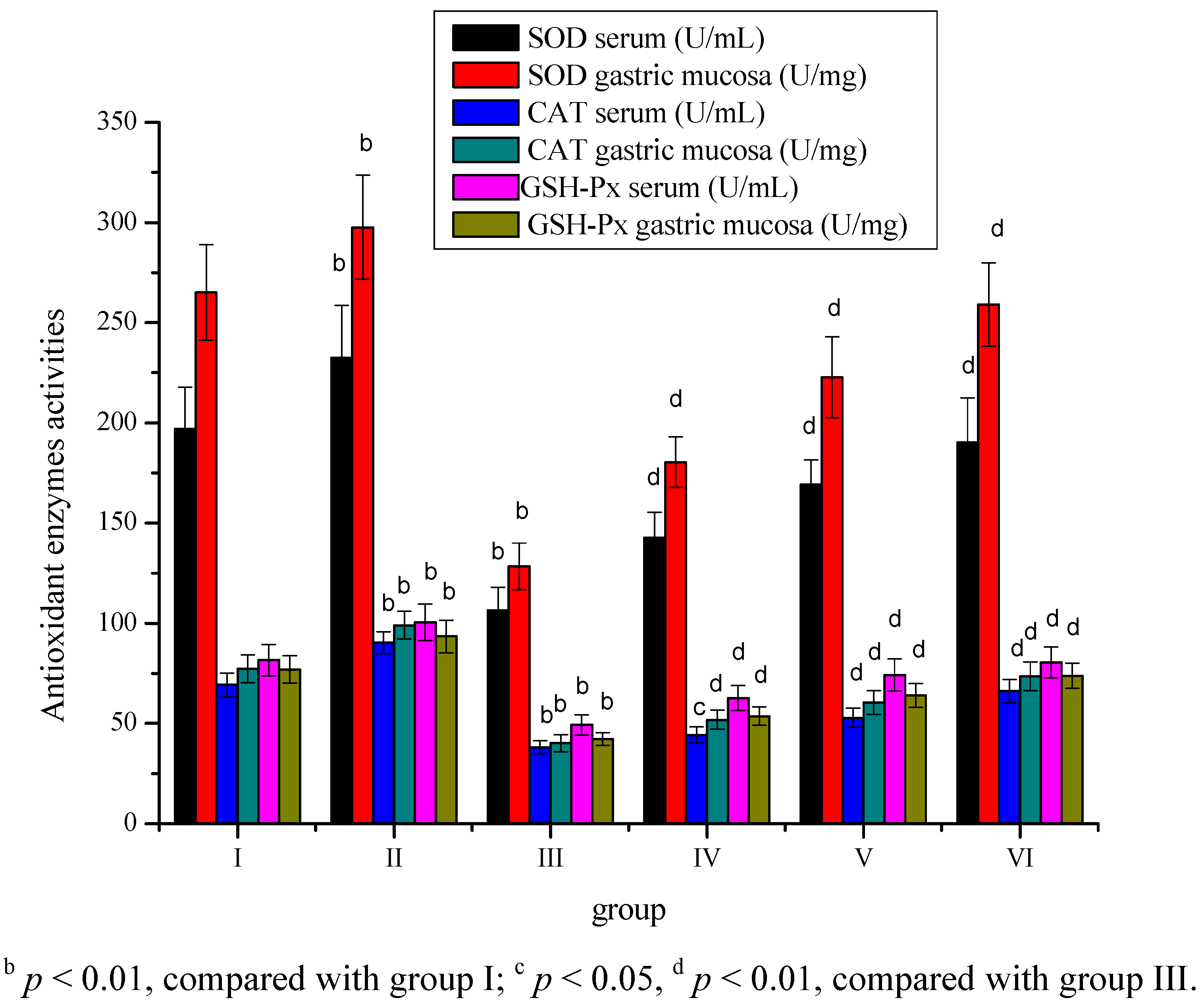
2. Results
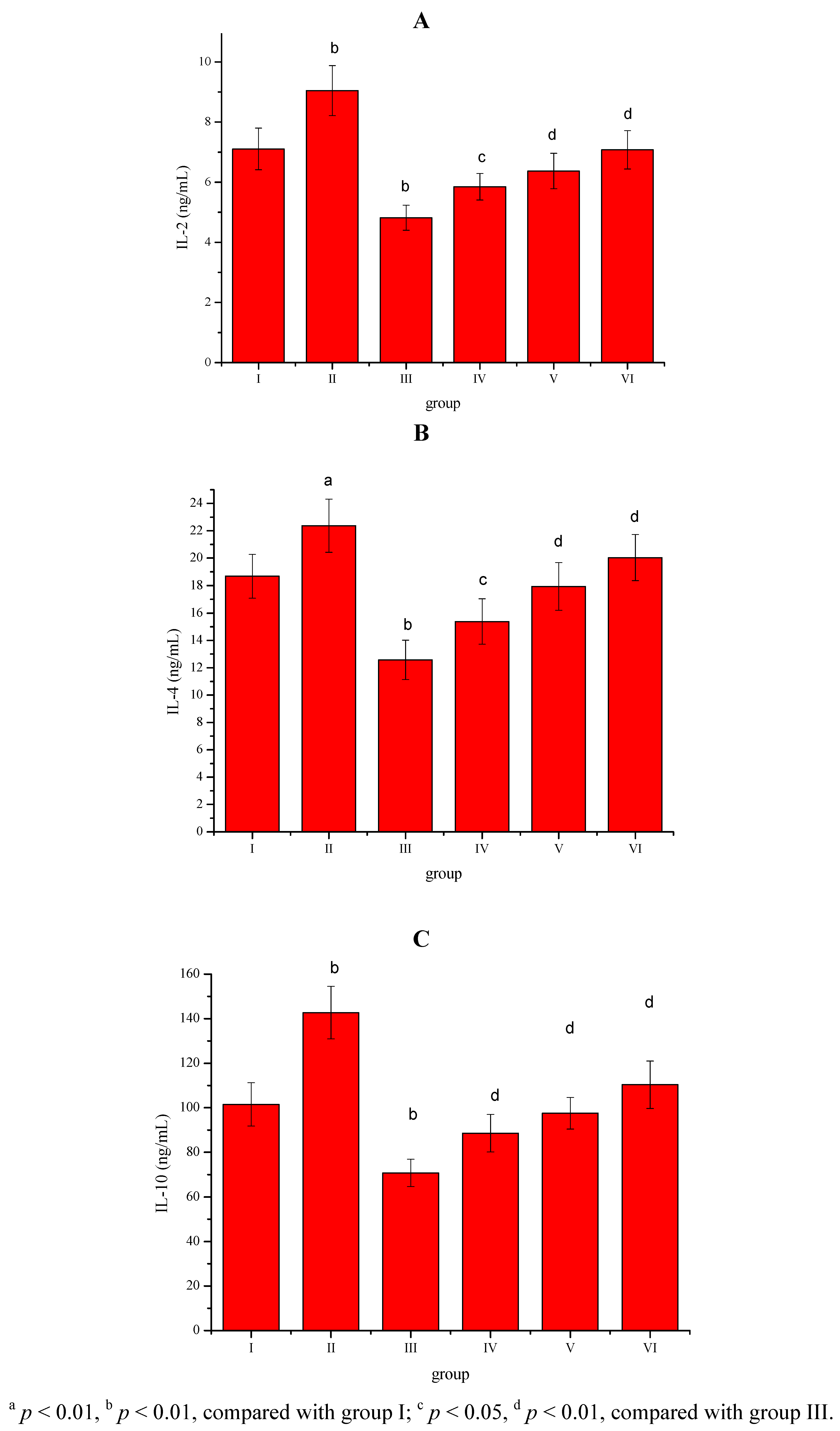
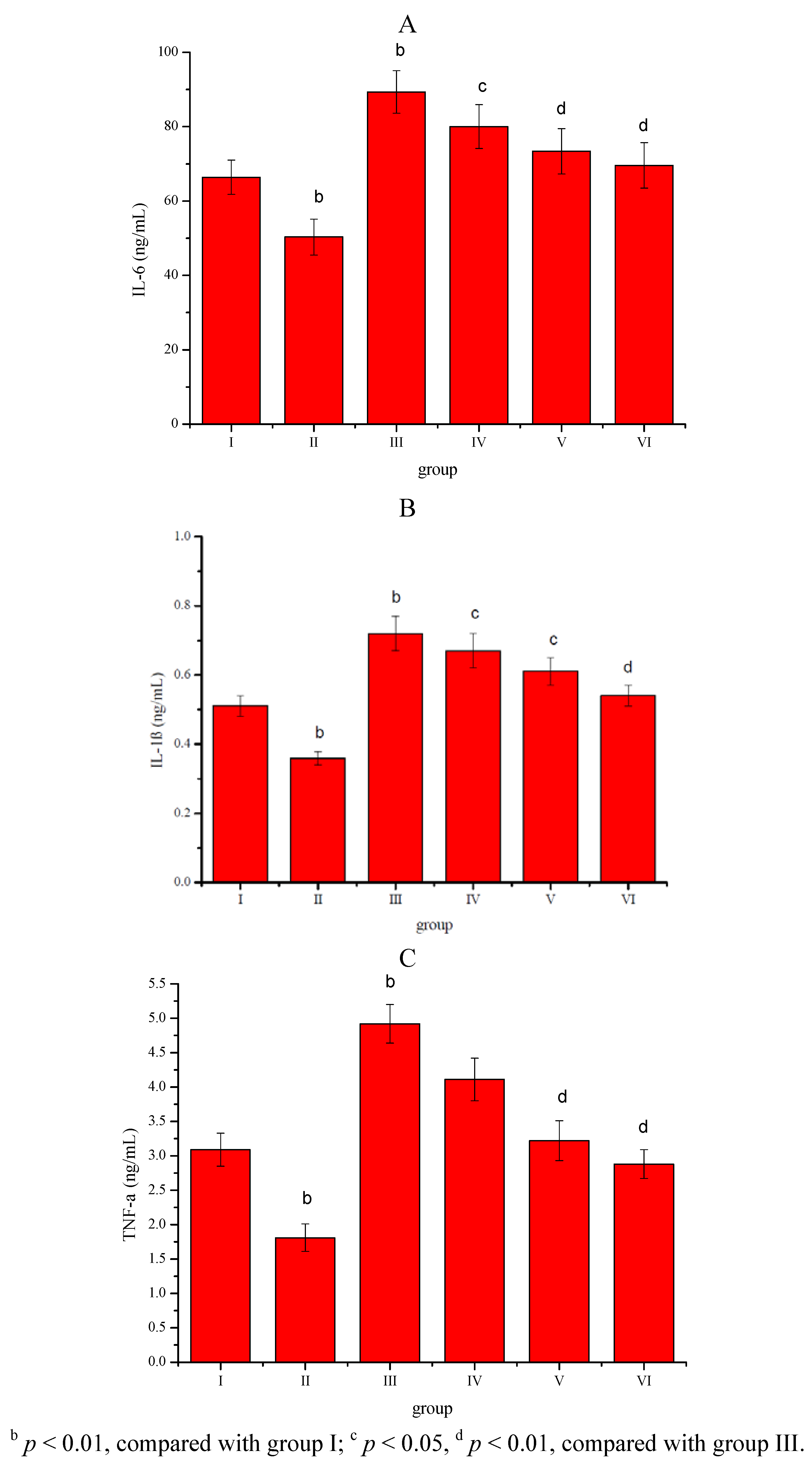
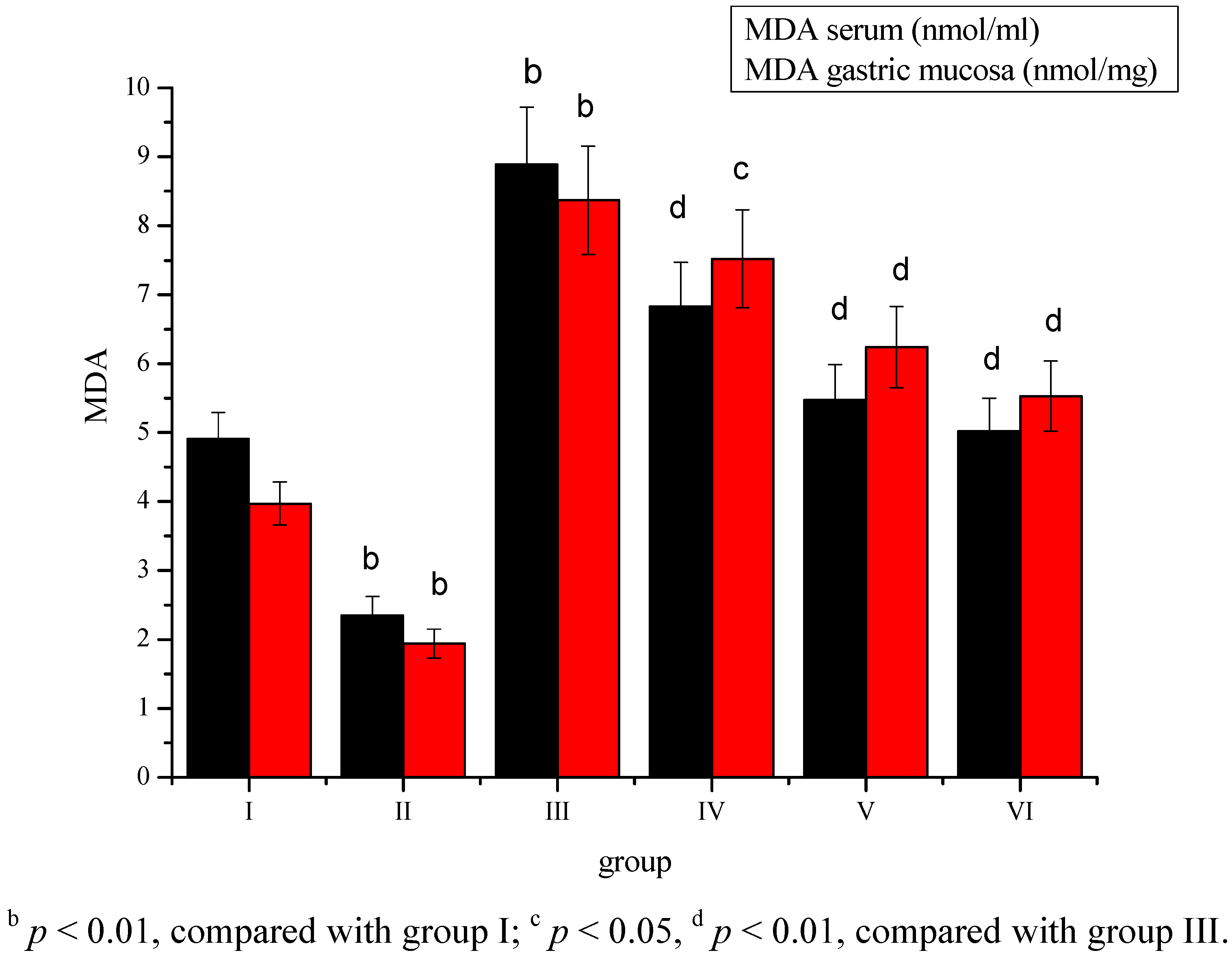
3. Discussion

4. Experimental
4.1. Materials
4.2. Preparation of Sargassum pallidum Aqueous Extract
4.3. Treatment of Animals
4.4. Biochemical Measurements
4.5. Statistical Analysis
5. Conclusions
- Sample Availability: Samples of Sargassum pallidum aqueous extract are available from the authors.
References
- Neugut, A.I.; Hayek, M.; Howe, G. Epidemiology of gastric cancer. Semin. Oncol. 1996, 3, 281–291. [Google Scholar]
- Block, G. Vitamin C and cancer prevention: The epidemiologic evidence. Am. J. Clin. Nutr. 1991, 53, 270–282. [Google Scholar]
- Debashis, B.; Kaushik, B.; Mrinnalini, B.; Russel, J.; Ranajit, K.B. Involvement of reactive oxygen species in gastric ulceration: Protection by melatonin. Indian J. Exp. Biol. 2002, 40, 693–705. [Google Scholar]
- Cadirci, E.; Suleyman, H.; Aksoy, H.; Halici, Z.; Ozgen, U.; Koc, A.; Ozturk, N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem. Biol. Interact. 2007, 170, 40–48. [Google Scholar] [CrossRef]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikkala, J. The inhibition of gastric mucosal injury by punicagranatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar]
- Anggadiredja, J.; Andyani, R.; Hayati, M. Antioxidant activity of Sargassum polycystum (Phaeophyta) and Laurencia obtusa (Rhodophyta) from Seribu Islands. J. Appl. Phycol. 1997, 9, 477–479. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar]
- Nahas, R.; Abatis, D.; Anagnostopoulou, M.A.; Kefalas, P.; Vagias, C.; Roussis, V. Radical-scavenging activity of aegean sea marine algae. Food Chem. 2007, 102, 577–581. [Google Scholar] [CrossRef]
- Zhang, W.W.; Duan, X.J.; Huang, H.L.; Zhang, Y.; Wang, B.G. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J. Appl. Phycol. 2007, 19, 97–108. [Google Scholar] [CrossRef]
- Blunden, G. Marine algae as sources of biologically active compounds. Interdiscipl. Sci. Rev. 1993, 18, 73–80. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Khomenko, V.A.; Ovodov, Yu.S. Polysaccharides of brown seaweeds VIII. The structure of the side chains of the sargassan molecule. Chem. Nat. Comp. 1975, 9, 96–97. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Svetashev, V.I. A high level of dihomogammalinolenic acid in brown alga Sargassum pallidum (Turn). Phytochemistry 1999, 50, 1209–1211. [Google Scholar]
- Fang, F.; Tang, Z.H. Study on the Antioxidant Activity of Polysaccharide from S. palladium. J. Anhui Agri. Sci. 2011, 39, 9590–9591. [Google Scholar]
- Guo, L.M.; Shao, C.L.; Liu, X.; Fang, Y.C.; Wei, Y.X.; Sun, L.L.; Gu, Q.Q.; Zhu, W.M.; Guan, H.S.; Wang, C.Y. Chemical composition of Sargassum pallidum and its in vitro antitumour activity. Chin. Tradit. Herbal Drug 2009, 40, 1879–1882. [Google Scholar]
- MacDermott, R.P. Chemokines in the inflammatory bowel diseases. J. Clin. Immunol. 1999, 19, 266–272. [Google Scholar] [CrossRef]
- Le, J.; Vilcek, J. Tumor necrosis factor and interleukin 1: Cytokines with multiple overlapping biological activities. Lab. Invest. 1987, 56, 248–324. [Google Scholar]
- Diamond, J.R.; Pesek, I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. Lab. Invest. 1991, 64, 21–28. [Google Scholar]
- Troost, E.; Hold, G.L.; Smith, M.G.; Chow, W.H.; Rabkin, C.S.; McColl, K.E.; El-Omar, E.M. The role of interleukin-1beta and other potential genetic markers as indicators of gastric cancer risk. Can. J. Gastroenterol. 2003, 17, 8–12. [Google Scholar]
- Brzozowski, T.; Konturek, P.Ch.; Konturek, S.J.; Drozdowicz, D.; Kwiecien, N.; Pajdo, R.; Bielanski, W.; Hahn, E.G. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia-reperfusion into gastric ulcers. Eur. J. Pharmacol. 2000, 398, 147–158. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Kwiecien, S.; Pajdo, R.; Karczewska, E.; Stachura, J. Water extracts of Helicobacter pylori delay healing of chronic gastric ulcers in rats: Role of cytokines and gastrin-somatostatin link. Digestion 1999, 60, 22–33. [Google Scholar]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Sliwowski, Z.; Drozdowicz, D.; Stachura, J.; Pajdo, R.; Hahn, E. Role of prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia-reperfusion-induced gastric lesions. Eur. J. Pharmacol. 1999, 385, 47–61. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Yoshida, N.; Kondo, M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig. Dis. Sci. 1998, 43, 30–34. [Google Scholar]
- Anderson, D. Antioxidant defences against reactive oxygen species causing genetic and other damage. Mutat. Res. 1996, 350, 103–108. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R.; Doelman, C.J. Oxidants and antioxidants: State of the art. Am. J. Med. 1991, 91, 2–13. [Google Scholar]
- Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Comporti, M. Lipid peroxidation and cellular damage in toxic liver injury. Lab. Invest. 1985, 53, 599–603. [Google Scholar]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikkala, J. The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Meth. Enzymol. 1990, 86, 421–431. [Google Scholar]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–168. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, R.-L.; Luo, W.-D.; Bi, T.-N.; Zhou, S.-K. Evaluation of Antioxidant and Immunity-Enhancing Activities of Sargassum pallidum Aqueous Extract in Gastric Cancer Rats. Molecules 2012, 17, 8419-8429. https://doi.org/10.3390/molecules17078419
Zhang R-L, Luo W-D, Bi T-N, Zhou S-K. Evaluation of Antioxidant and Immunity-Enhancing Activities of Sargassum pallidum Aqueous Extract in Gastric Cancer Rats. Molecules. 2012; 17(7):8419-8429. https://doi.org/10.3390/molecules17078419
Chicago/Turabian StyleZhang, Rui-Li, Wen-Da Luo, Tie-Nan Bi, and Shen-Kang Zhou. 2012. "Evaluation of Antioxidant and Immunity-Enhancing Activities of Sargassum pallidum Aqueous Extract in Gastric Cancer Rats" Molecules 17, no. 7: 8419-8429. https://doi.org/10.3390/molecules17078419
APA StyleZhang, R.-L., Luo, W.-D., Bi, T.-N., & Zhou, S.-K. (2012). Evaluation of Antioxidant and Immunity-Enhancing Activities of Sargassum pallidum Aqueous Extract in Gastric Cancer Rats. Molecules, 17(7), 8419-8429. https://doi.org/10.3390/molecules17078419



