Ipomoea aquatica Extract Shows Protective Action Against Thioacetamide-Induced Hepatotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Parameters of the Liver
| Group | ALP IU/L | ALT IU/L | AST IU/L | Protein g/L | Albumin g/L | Bilirubin g/L | Prothrombin time (PT) |
|---|---|---|---|---|---|---|---|
| Control (N.S) | 76.23 ± 0.47 | 37.8 ± 0.23 | 60.9 ± 0.75 | 68.65 ± 0.44 | 25.8 ± 0.32 | 1.23 ± 0.06 | 0.85 ± 0.01 |
| TAA+N.S | 234.1 ± 0.28 * | 160.8 ± 0.4 * | 221.63 ± 1.45 * | 48.88 ± 0.33 * | 8.68 ± 0.34 * | 4.73 ± 0.40 * | 1.67 ± 0.03 * |
| TAA+ Silymarin | 80.36 ± 0.65 ** | 38.13 ± 0.35 ** | 66.01 ± 0.27 ** | 66.81 ± 0.54 ** | 24.76 ± 0.53 ** | 1.24 ± 0.03 ** | 0.91 ± 0.01 ** |
| TAA+ I. aquatica 250mg/kg | 87.76 ± 0.43 ** | 83.83 ± 0.25 ** | 89.33 ± 0.57 ** | 63.03 ± 0.63 ** | 19.68 ± 0.34 ** | 2.42 ± 0.09 ** | 1.13 ± 0.02 ** |
| TAA+ I. aquatica 500 mg/kg | 77.8 ± 0.46 ** | 39.85 ± 0.33 ** | 63.73 ± 0.30 ** | 67.61 ± 0.27 ** | 24.93 ± 0.45 ** | 1.25 ± 0.03 ** | 0.90 ± 0.02 ** |
2.2. SOD, CAT and MDA Contents in Liver Homogenates
| Group | SOD | CAT | MDA |
|---|---|---|---|
| U/mg protein | U/mg protein | U/mg protein | |
| Control (N.S) | 18.03 ± 0.24 | 38.76 ± 0.32 | 1.19 ± 0.04 |
| TAA+N.S. | 8.63 ± 0.32 * | 18.73 ± 0.32 * | 4.80 ± 0.06 * |
| TAA+ Silymarin | 14.68 ± 0.21 ** | 37.13 ± 0.40 ** | 1.7 ± 0.04 ** |
| TAA+I. aquatica 250 mg/kg | 12.41 ± 0.27 ** | 26.46 ± 0.64 ** | 2.33 ± 0.09 ** |
| TAA+I. aquatica 500 mg/kg | 14.56 ± 0.29 ** | 37.95 ± 0.24 ** | 1.40 ± 0.09 ** |
2.3. Histopathological Study of the Liver
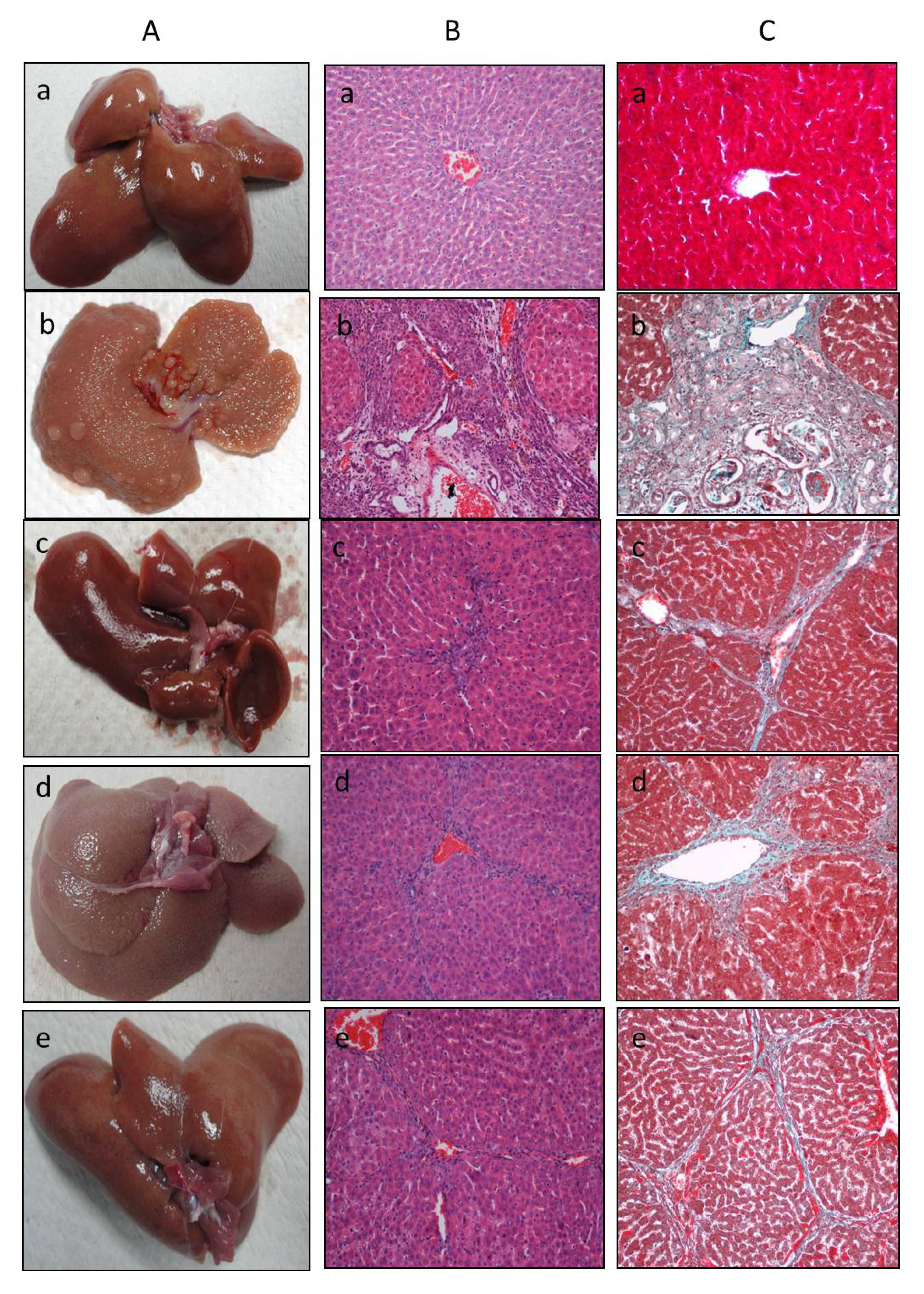
3. Experimental
3.1. Plant Material
3.2. Extraction Procedure
3.3. Experimental Animals
3.4. Chemicals
3.5. Acute Toxicity Test
3.6. Preparation of Doses
3.7. Protocol for Hepatoprotective Study
3.8. Assessment of Liver Function
3.9. Histopathological Examination
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Fu, H.; Xie, B.; Ma, S.; Zhu, X.; Fan, G.; Pan, S. Evaluation of antioxidant activities of principal carotenoids available in water spinach (Ipomoea aquatica). J. Food Compos. Anal. 2011, 24, 288–297. [Google Scholar] [CrossRef]
- Prasad, K.N.; Shivamurthy, G.R.; Aradhya, Z.S.M. Ipomoea aquatica, An Underutilized Green Leafy Vegetable: A Review. Int. J. Bot. 2008, 4, 123–129. [Google Scholar] [CrossRef]
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; The MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research (CSIR): New Delhi, India, 1956. [Google Scholar]
- Tseng, C.F.; Iwakami, S.; Mikajiri, A.; Shibuya, M.; Hanaoka, F.; Ebizuka, Y.; Padmawinata, K.; Sankawa, U. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-beta-phenethylamine and N-acyldopamine derivatives. Chem. Pharm. Bull. 1992, 40, 396–400. [Google Scholar] [CrossRef]
- Jain, A.; Roshnibala, S.; Kanjilal, P.B.; Singh, R.S.; Singh, H.B. Aquatic/semi-aquatic plants used in herbal remedies in the wetlands of Manipur, Northeastern India. Indian J. Tradit. Knowl. 2007, 6, 345–351. [Google Scholar]
- Malalavidhane, T.S.; Wickramasinghe, S.; Perera, M.S.A.; Jansz, E.R. Oral hypoglycaemic activity of Ipomoea aquatica in streptozotocin-induced, diabetic wistar rats and Type II diabetics. Phytother. Res. 2003, 17, 1098–1100. [Google Scholar] [CrossRef]
- Tee, E.; Lim, C.L. Carotenoid composition and content of Malaysian vegetables and fruits by the AOAC and HPLC methods. Food Chem. 1991, 41, 309–339. [Google Scholar] [CrossRef]
- Daniel, M. Polyphenols of some Indian vegetables. Curr. Sci. 1989, 58, 1332–1334. [Google Scholar]
- Tofern, B.; Mann, P.; Kaloga, M.; Jenett-Siems, K.; Witte, L.; Eich, E. Aliphatic pyrrolidine amides from two tropical convolvulaceous species. Phytochemistry 1999, 52, 1437–1441. [Google Scholar] [CrossRef]
- Samuel, A.J.S.J.; Mohan, S.; Chellappan, D.K.; Kalusalingam, A.; Ariamuthu, S. Hibiscus vitifolius (Linn.) root extracts shows potent protective action against anti-tubercular drug induced hepatotoxicity. J. Ethnopharmacol. 2012, 141, 396–402. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Aspollah Sukari, M.; Abdullah, R.; Taha, M.M.E.; Beng, N.K.; Isa, N.M. Typhonium flagelliforme inhibits the proliferation of murine leukemia WEHI-3 cells in vitro and induces apoptosis in vivo. Leukemia Res. 2010, 34, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Elhassan Taha, M.M.; Ibrahim, M.Y.; Syam, S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010, 131, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Syam, S.; Abdul, A.B.; Sukari, M.A.; Mohan, S.; Abdelwahab, S.I.; Wah, T.S. The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest. Molecules 2011, 16, 7155–7170. [Google Scholar] [CrossRef]
- Mangipudy, R.S.; Chanda, S.; Mehendale, H.M. Tissue repair response as a function of dose in thioacetamide hepatotoxicity. Environ. Health Persp. 1995, 260–267. [Google Scholar]
- Dorğru-Abbasoğlu, S.; Kanbağli, O.; Balkan, J.; Cevikbaş, U.; Aykaç-Tokerl, G.; Uysall, M. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum. Exp. Toxicol. 2001, 20, 23–27. [Google Scholar] [CrossRef]
- Ledda-Columbano, G.M.; Coni, P.; Curto, M.; Giacomini, L.; Faa, G.; Oliverio, S.; Piacentini, M.; Columbano, A. Induction of two different modes of cell death, apoptosis and necrosis, in rat liver after a single dose of thioacetamide. Am. J. Pathol. 1991, 139, 1099–1109. [Google Scholar]
- Okuyama, H.; Nakamura, H.; Shimahara, Y.; Uyama, N.; Kwon, Y.W.; Kawada, N.; Yamaoka, Y.; Yodoi, J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J. Hepatol. 2005, 42, 117–123. [Google Scholar]
- Moyer, V.A.; Ahn, C.; Sneed, S. Accuracy of clinical judgment in neonatal jaundice. Arch. Pediatr. Adolesc. Med. 2000, 154, 391–394. [Google Scholar]
- Woodman, D.D. Assessment of hepatotoxicity. In Animal Clinical Chemistry: A Primer for Toxicologist; Evans, G.O., Ed.; Taylor & Francis: London, UK, 1996. [Google Scholar]
- Zhao, J.; Su, Y.; Chen, A.; Yuan, H.; Liu, L.; Wu, W. Effect of Ginkgo Leaf Parenteral Solution on Blood and Cochlea Antioxidant and Immunity Indexes in OM Rats. Molecules 2011, 16, 10433–10442. [Google Scholar] [CrossRef]
- Georgieva, N.; Gadjeva, V.; Tolekova, A. New isonicotinoylhydrazones with SSA protect against oxidative-hepatic injury of isoniazid. TJS 2004, 2, 37–43. [Google Scholar]
- Tribble, D.L.; Aw, T.Y.; Jones, D.P. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology 1987, 7, 377–386. [Google Scholar] [CrossRef]
- Irani, A.N.; Malhi, H.; Slehria, S.; Gorla, G.R.; Volenberg, I.; Schilsky, M.L.; Gupta, S. Correction of liver disease following transplantation of normal rat hepatocytes into Long-Evans Cinnamon rats modeling Wilson's disease. Mol. Ther. 2001, 3, 302–309. [Google Scholar] [CrossRef]
- AydIn, A.F.; Küskü-Kiraz, Z.; Doğru-Abbasoğlu, S.; Güllüoğlu, M.; Uysal, M.; Koçak-Toker, N. Effect of carnosine against thioacetamide-induced liver cirrhosis in rat. Peptides 2010, 31, 67–71. [Google Scholar] [CrossRef]
- Bastway Ahmed, M.; Hasona, N.A.; Selemain, A.H. Protective Effects of Extract from Dates (Phoenix Dactylifera L.) and Ascorbic Acid on Thioacetamide-Induced Hepatotoxicity in Rats. Iran. J. Pharm. Res. 2010, 7, 193–201. [Google Scholar]
- Mahmood, A.A.; Mariod, A.A.; Abdelwahab, S.I.; Ismail, S.; Al-Bayaty, F. Potential activity of ethanolic extract of Boesenbergia rotunda (L.) rhizomes extract in accelerating wound healing in rats. J. Med. Plants Res. 2010, 4, 1570–1576. [Google Scholar]
- Wang, H.; Wei, W.; Wang, N.P.; Gui, S.Y.; Wu, L.; Sun, W.Y.; Xu, S.Y. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005, 77, 1902–1915. [Google Scholar] [CrossRef]
- Yasuda, M.; Shimizu, I.; Shiba, M.; Ito, S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 1999, 29, 719–727. [Google Scholar] [CrossRef]
- Al-Bayaty, F.; Abdulla, M.; Abu Hassan, M.I.; Masud, M.I. Wound healing potential by hyaluronate gel in streptozotocin-induced diabetic rats. Sci. Res. Essays 2010, 5, 2756–2760. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alkiyumi, S.S.; Abdullah, M.A.; Alrashdi, A.S.; Salama, S.M.; Abdelwahab, S.I.; Hadi, A.H.A. Ipomoea aquatica Extract Shows Protective Action Against Thioacetamide-Induced Hepatotoxicity. Molecules 2012, 17, 6146-6155. https://doi.org/10.3390/molecules17056146
Alkiyumi SS, Abdullah MA, Alrashdi AS, Salama SM, Abdelwahab SI, Hadi AHA. Ipomoea aquatica Extract Shows Protective Action Against Thioacetamide-Induced Hepatotoxicity. Molecules. 2012; 17(5):6146-6155. https://doi.org/10.3390/molecules17056146
Chicago/Turabian StyleAlkiyumi, Salim Said, Mahmood Ameen Abdullah, Ahmed Salim Alrashdi, Suzy Munir Salama, Siddig Ibrahim Abdelwahab, and A. Hamid A. Hadi. 2012. "Ipomoea aquatica Extract Shows Protective Action Against Thioacetamide-Induced Hepatotoxicity" Molecules 17, no. 5: 6146-6155. https://doi.org/10.3390/molecules17056146




