Synthesis of N-substituted Acridinediones and Polyhydroquinoline Derivatives in Refluxing Water
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Typical Procedure for the Synthesis of Acridinediones: 3,3,6,6-Tetramethyl-9,10-diphenyl-3,4,6,7,9,10-hexahydro-1,8-(2H,5H)-acridinedione (5a)
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Stilo, A.D.; Visentin, S.; Cena, C.; Gasco, A.M.; Ermondi, G.; Gasco, A. New 1,4-dihydropyridines conjugated to furoxanyl moieties, endowed with both nitric oxide-like and calcium channel antagonist vasodilator activities. J. Med. Chem. 1998, 41, 5393–5401. [Google Scholar] [CrossRef] [PubMed]
- Janis, R.A.; Triggle, D.J. New developments in Ca2+ channel antagonists. J. Med. Chem. 1993, 26, 775–785. [Google Scholar] [CrossRef]
- Kaya, M.; Yıldırır, Y.; Türker, L. Synthesis and laser activity of halo-acridinedione derivatives. J. Heterocyclic Chem. 2009, 46, 294–297. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Arzroomchilar, E. 1-Methylimidazolium triflouroacetate ([Hmim]TFA): An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Catal. Commun. 2008, 9, 939–942. [Google Scholar] [CrossRef]
- Shi, D.Q.; Ni, S.N.; Yang, F.; Shi, J.W.; Dou, G.L.; Li, X.Y.; Wang, X.S. An efficient synthesis of polyhydroacridine derivatives by the three-component reaction of aldehydes, amines and dimedone in ionic liquid. J. Heterocycl. Chem. 2008, 45, 653–660. [Google Scholar] [CrossRef]
- Kumara, D.; Sandhu, J.S. Efficient, solvent-free, microwave-enhanced condensation of 5,5-dimethyl-1,3-cyclohexanedione with aldehydes and imines using libr as inexpensive, mild catalyst. Synth. Commun. 2010, 40, 510–517. [Google Scholar] [CrossRef]
- Venkatesan, K.; Pujari, S.S.; Srinivasan, K.V. Proline-catalyzed simple and efficient synthesis of 1,8-dioxo-decahydroacridines in aqueous ethanol medium. Synth. Commun. 2009, 39, 228–241. [Google Scholar] [CrossRef]
- Niknam, K.; Panahi, F.; Saberi, D.; Mohagheghnejad, M. Silica-bonded S-sulfonic acid as recyclable catalyst for the synthesis of 1,8-dioxo-decahydroacridines and 1,8-dioxo-octahydroxanthenes. J. Heterocycl. Chem. 2010, 47, 292–300. [Google Scholar]
- Kidwai, M.; Bhatnagar, D. Ceric ammonium nitrate (CAN) catalyzed synthesis of n-substituted decahydroacridine-1,8-diones in PEG. Tetrahedron Lett. 2010, 51, 2700–2703. [Google Scholar] [CrossRef]
- Shi, D.Q.; Shi, J.W.; Yao, H. Clean synthesis of 9,10-diarylacridine derivatives in aqueous media. Chin. J. Org. Chem. 2009, 29, 239–244. [Google Scholar]
- Shi, D.Q.; Mou, J.; Zhuang, Q.Y.; Wang, X.S. Synthesis of ethyl 2,7,7-trimethyl-5-oxo-4-aryl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate in water. Chin. J. Org. Chem. 2004, 24, 1569–1572. [Google Scholar]
- Ramazani, A.; Rezaei, A. Novel one-pot, four-component condensation reaction: An efficient approach for the synthesis of 2,5-disubstituted 1,3,4-oxadiazole derivatives by a Ugi-4CR/aza-wittig sequence. Org. Lett. 2010, 12, 2852–2855. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, A.; Karimi, Z.; Souldozi, A.; Ahmadi, Y. Four-component synthesis of 1,3,4-oxadiazole derivatives from n-isocyaniminotriphenylphosphorane, aromatic carboxylic acids, aromatic bis-aldehydes, and secondary amines. Turk. J. Chem. 2012, 36, 81–91. [Google Scholar]
- Ramazani, A.; Rouhani, M.; Rezaei, A.; Shajari, N.; Souldozi, A. The reaction of (n-isocyanimino)triphenylphosphorane with biacetyl in the presence of aromatic carboxylic acids: Efficient one-pot three-component reaction for the synthesis of 3-(5-aryl-1,3,4-oxadiazol-2-yl)-3-hydroxybutan-2-one derivatives. Helv. Chim. Acta 2011, 94, 282–288. [Google Scholar] [CrossRef]
- Wang, G.W.; Xia, J.J.; Miao, C.B.; Wu, X.L. Environmentally friendly and efficient synthesis of various 1,4-dihydropyridines in pure water. Bull. Chem. Soc. Jpn. 2006, 79, 454–459. [Google Scholar] [CrossRef]
- Wang, G.W.; Lu, Q.Q.; Xia, J.J. Three types of products obtained unexpectedly from the reaction of dimedone with chalcones. Eur. J. Org. Chem. 2011, 4429–4438. [Google Scholar] [CrossRef]
- Luo, H.; Kang, Y.; Nie, H.; Yang, L. Fe3+-montmorillonite: An efficient solid catalyst for one-pot synthesis of decahydroacridine derivatives. J. Chin. Chem. Soc. 2008, 55, 1280–1285. [Google Scholar] [CrossRef]
- Shi, D.Q.; Shi, J.W.; Yao, H. Three-component one-pot synthesis of polyhydroacrodine derivatives in aqueous media. Synth. Commun. 2009, 39, 664–675. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Saidi Reddy, V.; Rao, Y.K. Amberlyst-15: An efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. J. Molecular Catal. A: Chem. 2006, 247, 233–239. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Chen, Y.; Liu, C.N.; Cai, K.Y.; Tu, S.J. A green procedure for the synthesis of 1,8-dioxodecahydroacridine derivatives under microwave irradiation in aqueous media without catalyst. J. Heterocycl. Chem. 2010, 47, 363–367. [Google Scholar]
- Shen, Y.B.; Wang, G.W. Solvent-free synthesis of xanthenediones and acridinediones. ARKIVOC 2008, xvi, 1–8. [Google Scholar]
- Tu, S.; Li, T.; Zhang, Y.; Shi, F.; Xu, J.; Wang, Q.; Zhang, J.; Zhu, X.; Jiang, B.; Jia, R.; Zhang, J. New reaction of schiff base with dimedone: New method for the acridine derivatives under microwave irradiation. J. Heterocycl. Chem. 2007, 44, 83–88. [Google Scholar] [CrossRef]
- Shen, W.; Wang, L.M.; Tian, H.; Tang, J.; Yu, J.J. Brønsted acidic imidazolium salts containing perfluoroalkyl tails catalyzed one-pot synthesis of 1,8-dioxo-decahydroacridines in water. J. Fluorine Chem. 2009, 130, 522–527. [Google Scholar] [CrossRef]
- Bandgar, B.P.; More, P.E.; Kamble, V.T.; Totre, J.V. Synthesis of polyhydroquinoline derivatives under aqueous media. ARKIVOC 2008, xv, 1–8. [Google Scholar]
Sample Availability: Samples of the compounds 5a–n, 7a–d are available from the authors. |

| Entry | Amine | Reaction time/min | Product | R | Yield / % a |
|---|---|---|---|---|---|
| 1 | NH4OAc | 40 min | 4a | H | 86 |
| 2 | NH4HCO3 | 40 min | 4b | H | 83 |
| 3 | C6H5-NH2 | 90 min | 4c | C6H5 | 71 |
| 4 | 4-CH3-C6H5-NH2 | 90 min | 4d | 4-CH3-C6H4 | 69 |
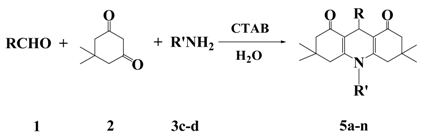
| Entry | R | R’ | Product | Yield / % a | m.p. (lit.) / °C |
|---|---|---|---|---|---|
| 1 | C6H5 | C6H5 | 5a | 80 | 220–222 (200–205) [17] |
| 2 | 4-Cl-C6H4 | C6H5 | 5b | 86 | 243–245 (233–235) [18] |
| 3 | 4-CN-C6H4 | C6H5 | 5c | 88 | 265–267 |
| 4 | 4-NO2-C6H4 | C6H5 | 5d | 85 | 281–282 (216–218) [17] |
| 5 | 3- NO2-C6H4 | C6H5 | 5e | 84 | 272–274 (276–278) [19] |
| 6 | 3,4- Cl2-C6H3 | C6H5 | 5f | 90 | 274–275 |
| 7 | 4-CH3O-C6H4 | C6H5 | 5g | 70 | 291–293 (290–291) [20] |
| 8 | C6H5 | 4-CH3-C6H4 | 5h | 81 | 261–263 (264–266) [21] |
| 9 | 4-Cl-C6H4 | 4-CH3-C6H4 | 5i | 85 | 270–272 (271–272) [21] |
| 10 | 4-CN-C6H4 | 4-CH3-C6H4 | 5j | 88 | 268–270 (273–275) [21] |
| 11 | 4-NO2-C6H4 | 4-CH3-C6H4 | 5k | 83 | >300 (>300) [22] |
| 12 | 3-NO2-C6H4 | 4-CH3-C6H4 | 5l | 85 | 281–283 (283–284) [23] |
| 13 | 3,4-Cl2-C6H3 | 4-CH3-C6H4 | 5m | 88 | 253–255 (250–252) [23] |
| 14 | 4-CH3O-C6H4 | 4-CH3-C6H4 | 5n | 72 | 280–282 (281–283) [23] |
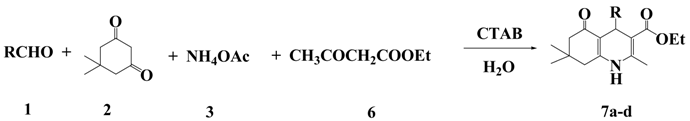
| Entry | R | Product | Yield / % a | m.p. (lit.) / °C |
|---|---|---|---|---|
| 1 | C6H5 | 7a | 85 | 224–226 (228–229) [24] |
| 2 | 4-CH3O-C6H4 | 7b | 81 | 257–259 (260–262) [24] |
| 3 | 4-Cl-C6H4 | 7c | 90 | 244–266 (245–246) [24] |
| 4 | 4-NO2-C6H4 | 7d | 88 | 242–244 (241–242) [24] |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xia, J.-J.; Zhang, K.-H. Synthesis of N-substituted Acridinediones and Polyhydroquinoline Derivatives in Refluxing Water. Molecules 2012, 17, 5339-5345. https://doi.org/10.3390/molecules17055339
Xia J-J, Zhang K-H. Synthesis of N-substituted Acridinediones and Polyhydroquinoline Derivatives in Refluxing Water. Molecules. 2012; 17(5):5339-5345. https://doi.org/10.3390/molecules17055339
Chicago/Turabian StyleXia, Jing-Jing, and Ke-Hua Zhang. 2012. "Synthesis of N-substituted Acridinediones and Polyhydroquinoline Derivatives in Refluxing Water" Molecules 17, no. 5: 5339-5345. https://doi.org/10.3390/molecules17055339




