Isolation and Identification of Myo-Inositol Crystals from Dragon Fruit (Hylocereus polyrhizus)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Crystallization


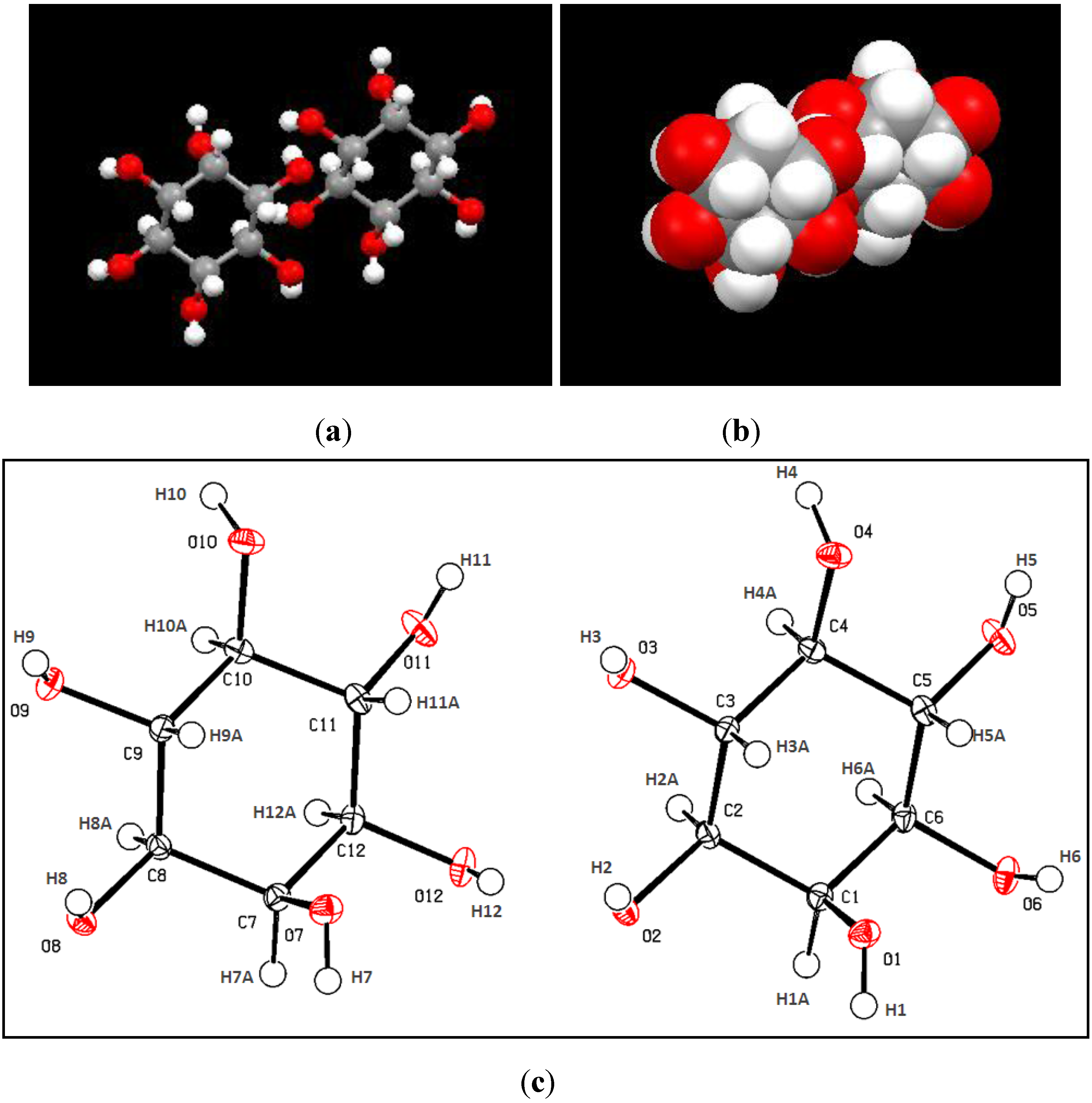
2.2. X-Ray Crystallograhy Analysis
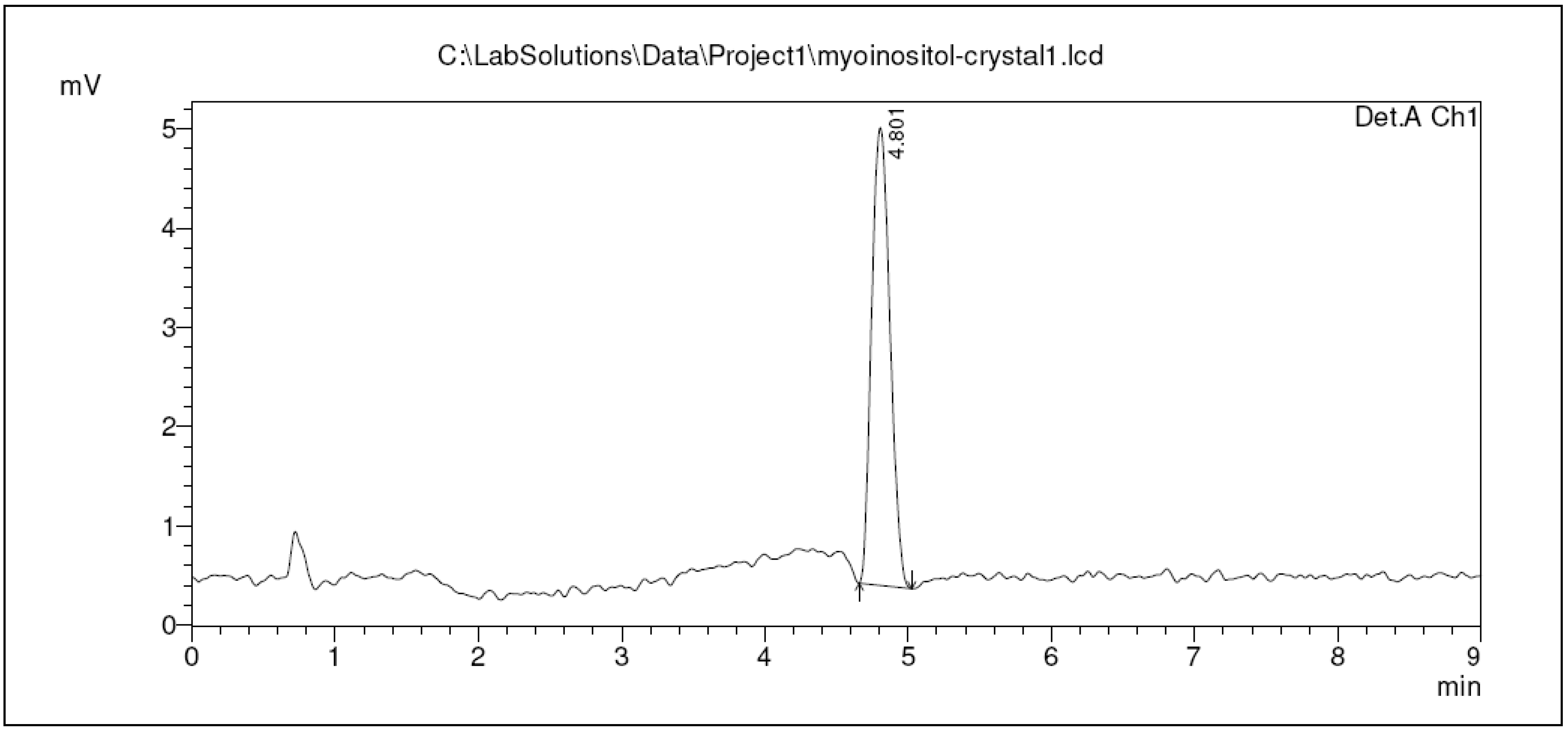
2.3. Qualification of Crystal Purity Using High Performance Liquid Chromatography
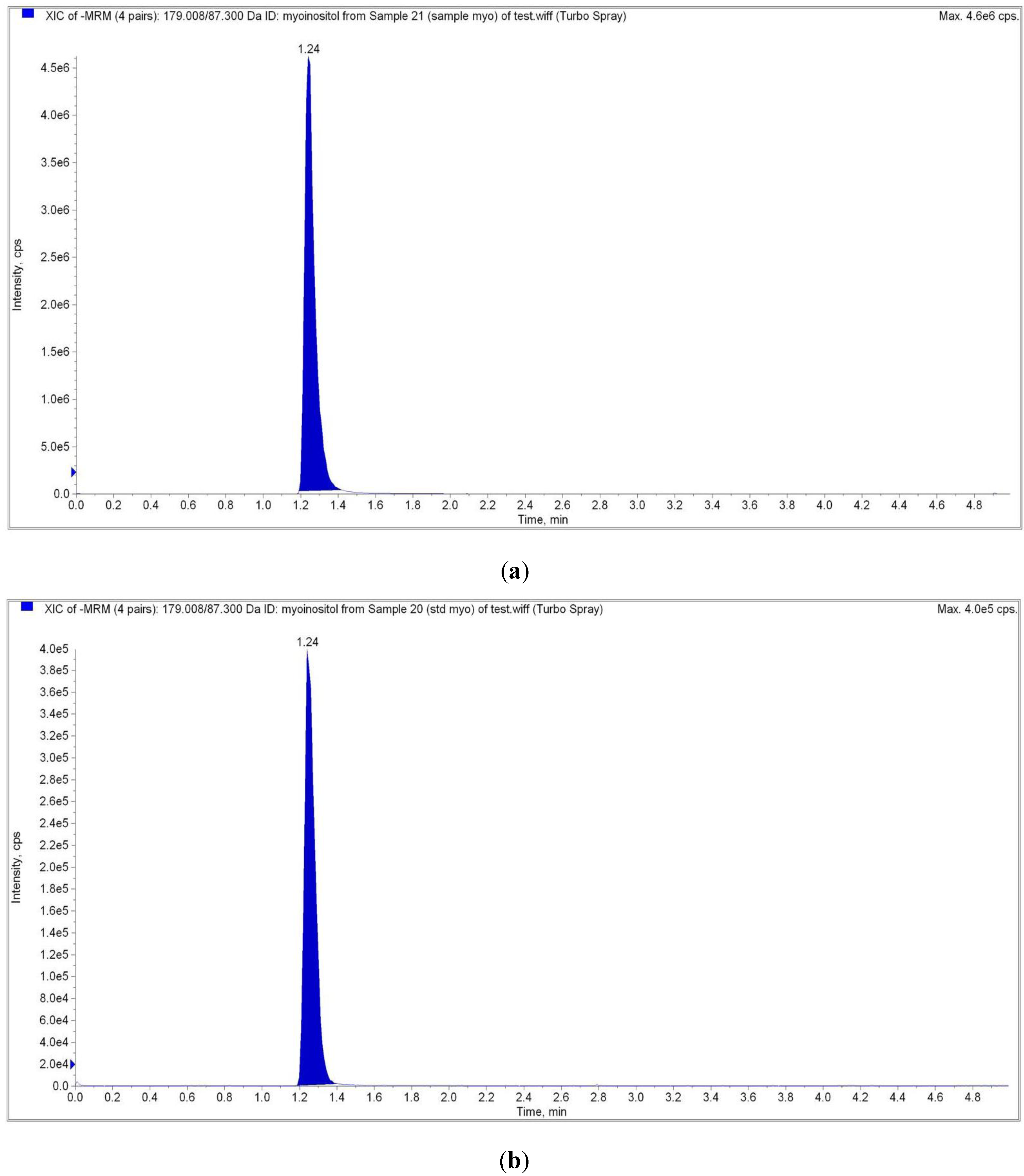
2.4. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Analysis
| Analyte Peak Name | Analyte RT | Precursor ion (M/Z) | Product Ion (M/Z) |
|---|---|---|---|
| Sample | 1.24 | 179.00 | 87.00 |
| Myo-inositol Standard | 1.24 | 179.00 | 87.00 |
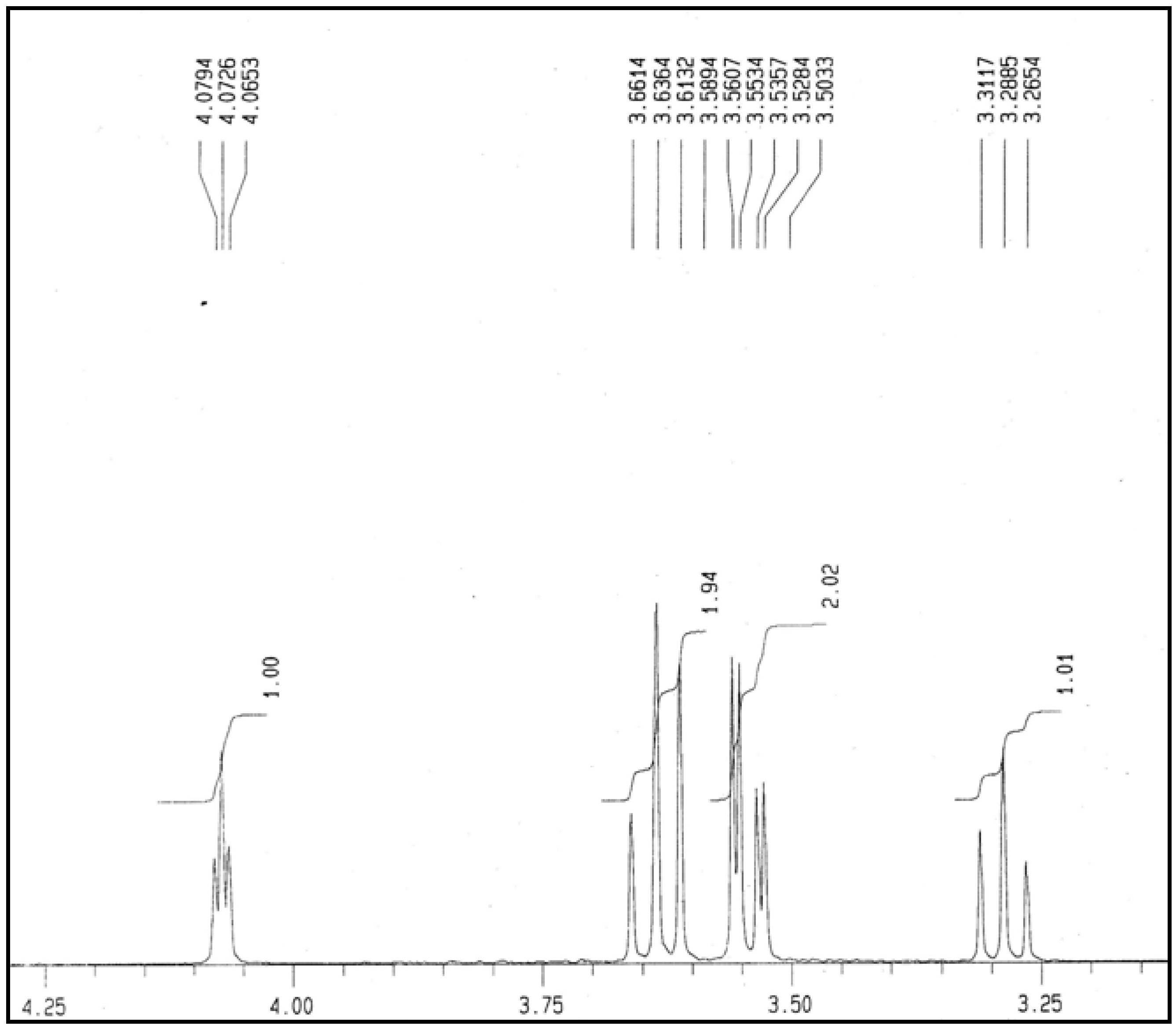
2.5. NMR Analysis
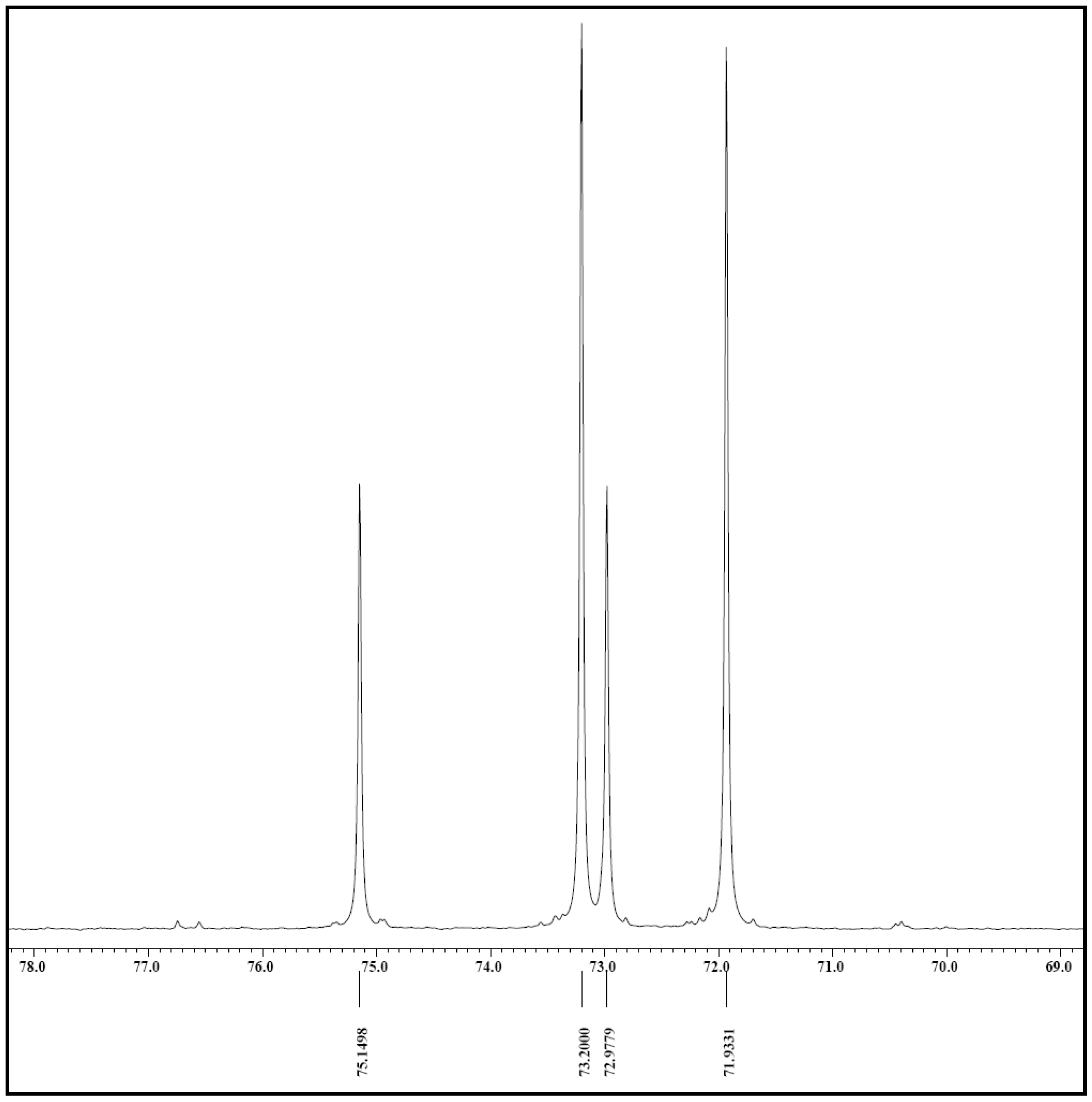
3. Experimental
3.1. Sample Preparation
3.2. Purification
3.3. Crystallization
3.4. Crystal structure Determination Using X-Ray Crystallography
3.5. Qualification of Crystal Purity Using High Performance Liquid Chromatography
3.6. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Analysis
3.7. Nuclear Magnetic Resonance (NMR) Analysis
4. Conclusions
Acknowledgments
References and Notes
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red purple pitaya, Hylocereus polyrhizus (Weber) Britton and Rose. Food Chem. 2002, 77, 101–106. [Google Scholar] [CrossRef]
- le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Rebecca, O.P.S.; Zuliana, R.; Boyce, A.N.; Chandran, S. Determining pigment extraction efficiency and pigment stability of dragon fruit (Hylocereus polyrhizus). J. Biol. Sci. 2008, 8, 1174–1180. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar]
- Rebecca, O.P.S.; Boyce, A.N.; Chandran, S. Pigment identification and antioxidant properties of red dragon fruit (Hylocereus polyrhizus). Afr. J. Biotech. 2010, 9, 1450–1454. [Google Scholar]
- Larner, J. D-chiro-inositol—Its functional role in insulin action and its deficit in insulin resistance. Int. J. Exp. Diabetes Res. 2002, 3, 47–60. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Whiting, P.H.; Palmano, K.P.; Hawthorne, J.N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem. J. 1979, 179, 549–553. [Google Scholar]
- Barrientos, L.G.; Murthy, P.P.P. Conformational studies of myo-inositol phosphates. Carbohydr. Res. 1996, 296, 39–54. [Google Scholar] [CrossRef]
- Silverstone, P.H.; McGrath, B.M.; Kim, H. Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005, 7, 1–10. [Google Scholar]
- Solomonia, R.; Mikautadze, E.; Nozadze, M.; Kuchiashvili, N.; Lepsveridze, E.; Kiguradze, T. Myo-inositol treatment prevents biochemical changes triggered by kainate-induced status epilepticus. Neurosci. Lett. 2010, 468, 277–281. [Google Scholar] [CrossRef]
- Giordano, D.; Corrado, F.; Santamaria, A.; Quattrone, S.; Pintaudi, B.; di Benedetto, A.; D’Anna, R. Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: A perspective, randomized, placebo-controlled study. Menopause 2011, 18, 102–104. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Afzal, A.J.; Yaegashi, S.; Ruben, E.; Triwitayakorn, K.; Njiti, V.N.; Ahsan, R.; Wood, A.J.; Lightfoot, D.A. A pyramid of loci for partial resistance to Fusarium solani f. sp. glycines maintains Myo-inositol-1-phosphate synthase expression in soybean roots. Theor. Appl. Genet. 2002, 105, 1115–1123. [Google Scholar] [CrossRef]
- Deranieh, R.M.; Greenberg, M.L. Cellular consequences of inositol depletion. Biochem. Soc. Trans. 2009, 37, 1099–1103. [Google Scholar] [CrossRef]
- Brex, P.A.; Gomez-Anson, B.; Parker, G.J.; Molyneux, P.D.; Miszkiel, K.A.; Barker, G.J.; MacManus, D.G.; Davie, C.A.; Plant, G.T.; et al. Proton MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis. J. Neurol. Sci. 1999, 166, 16–22. [Google Scholar] [CrossRef]
- McLaurin, J.; Franklin, T.; Chakrabartty, A.; Fraser, P.E. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-β fibril growth and arrest. J. Mol. Biol. 1998, 278, 183–194. [Google Scholar] [CrossRef]
- Thomas, T.P.; Porcellati, F.; Kato, K.; Stevens, M.J.; Sherman, W.R.; Greene, D.A. Effects of glucose on sorbitol pathway activation, cellular redox, and metabolism of myo-Inositol, phosphoinositide, and diacylglycerol in cultured human retinal pigment epithelial ce. J. Clin. Invest. 1994, 93, 2718–2724. [Google Scholar] [CrossRef]
- Rabinowitz, I.N.; Kraut, J. The crystal structure of myo-inositol. Acta Crystallogr. 1964, 17, 159–168. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Hulme, A.N.; McNab, H.; Quye, A. The natural constituents of historical textile dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar]
- Winnik, W.M.; Kitchin, K.T. Measurement of oxidative stress parameters using liquid chromatography–tandem mass spectroscopy (LC-MS/MS). Toxicol. App. Pharmacol. 2008, 233, 100–106. [Google Scholar] [CrossRef]
- Kindt, E.; Shum, Y.; Badura, L.; Snyder, P.J.; Brant, A.; Fountain, S.; Szekely-Klepser, G. Development and validation of an LC/MS/MS procedure for the quantification of endogenous myo-inositol concentrations in rat brain tissue homogenates. Anal. Chem. 2004, 76, 4901–4908. [Google Scholar]
- de Jong, W.H.A.; de Vries, E.G.E.; Kema, I.P. Current status and future developments of LC-MS/MS in clinical chemistry for quantification of biogenic amines. Clin. Biochem. 2011, 44, 95–103. [Google Scholar]
- Cerdan, S.; Parrilla, R.; Santoro, J.; Rico, M. lH-NMR detection of cerebral myo-inositol. FEBS Lett. 1985, 187, 167–172. [Google Scholar] [CrossRef]
- Harwood, A.J. Lithium and bipolar mood disorder: The inositol-depletion hypothesis revisited. Mol. Psychiatr. 2005, 10, 117–126. [Google Scholar] [CrossRef]
- Shimon, H.; Agam, G.; Belmaker, R.H.; Hyde, T.M.; Kleinman, J.E. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am. J. Psychiatr. 1997, 154, 1148–1150. [Google Scholar]
- Clements, R.S.J.; Reynertson, R. Myoinositol metabolism in diabetes mellitus. Effect of insulin treatment. Diabetes 1977, 26, 215–221. [Google Scholar]
- Fux, M.; Levine, J.; Aviv, A.; Belmaker, R.H. Inositol treatment of obsessive-compulsive disorder. Am. J. Psychiatr. 1996, 153, 1219–1221. [Google Scholar]
- Clements, R.S.J.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar]
- Ishitani, M.; Majumder, A.L.; Bornhouser, A.; Michalowski, C.B.; Jensen, R.C.; Bohnert, H.J. Coordinate transcriptional induction of myo-inositol metabolism during environmental stress. Plant J. 1996, 9, 537–548. [Google Scholar]
- RayChaudhuri, A.; Majumber, A.L. Salinity-induced enhancement of 1-myo-inositol 1-phosphate synthase in rice (Oryza sativa L.). Plant Cell Environ. 1996, 19, 1437–1442. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar]
- Saito, N.; Hirata, K.; Hotta, R.; Hayashi, K. Isolation and crystallization of genuine red anthocyanins. Proc. Jpn. Acad. 1964, 40, 516–521. [Google Scholar]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar]
- Sheldrick, G.M. SHELX97-Programs for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the myo-inositol crystal as seen in Figure 2 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rebecca, O.P.S.; Boyce, A.N.; Somasundram, C. Isolation and Identification of Myo-Inositol Crystals from Dragon Fruit (Hylocereus polyrhizus). Molecules 2012, 17, 4583-4594. https://doi.org/10.3390/molecules17044583
Rebecca OPS, Boyce AN, Somasundram C. Isolation and Identification of Myo-Inositol Crystals from Dragon Fruit (Hylocereus polyrhizus). Molecules. 2012; 17(4):4583-4594. https://doi.org/10.3390/molecules17044583
Chicago/Turabian StyleRebecca, Ow Phui San, Amru Nasrulhaq Boyce, and Chandran Somasundram. 2012. "Isolation and Identification of Myo-Inositol Crystals from Dragon Fruit (Hylocereus polyrhizus)" Molecules 17, no. 4: 4583-4594. https://doi.org/10.3390/molecules17044583





