Enzymatic Activity Enhancement of Non-Covalent Modified Superoxide Dismutase and Molecular Docking Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Activity Results
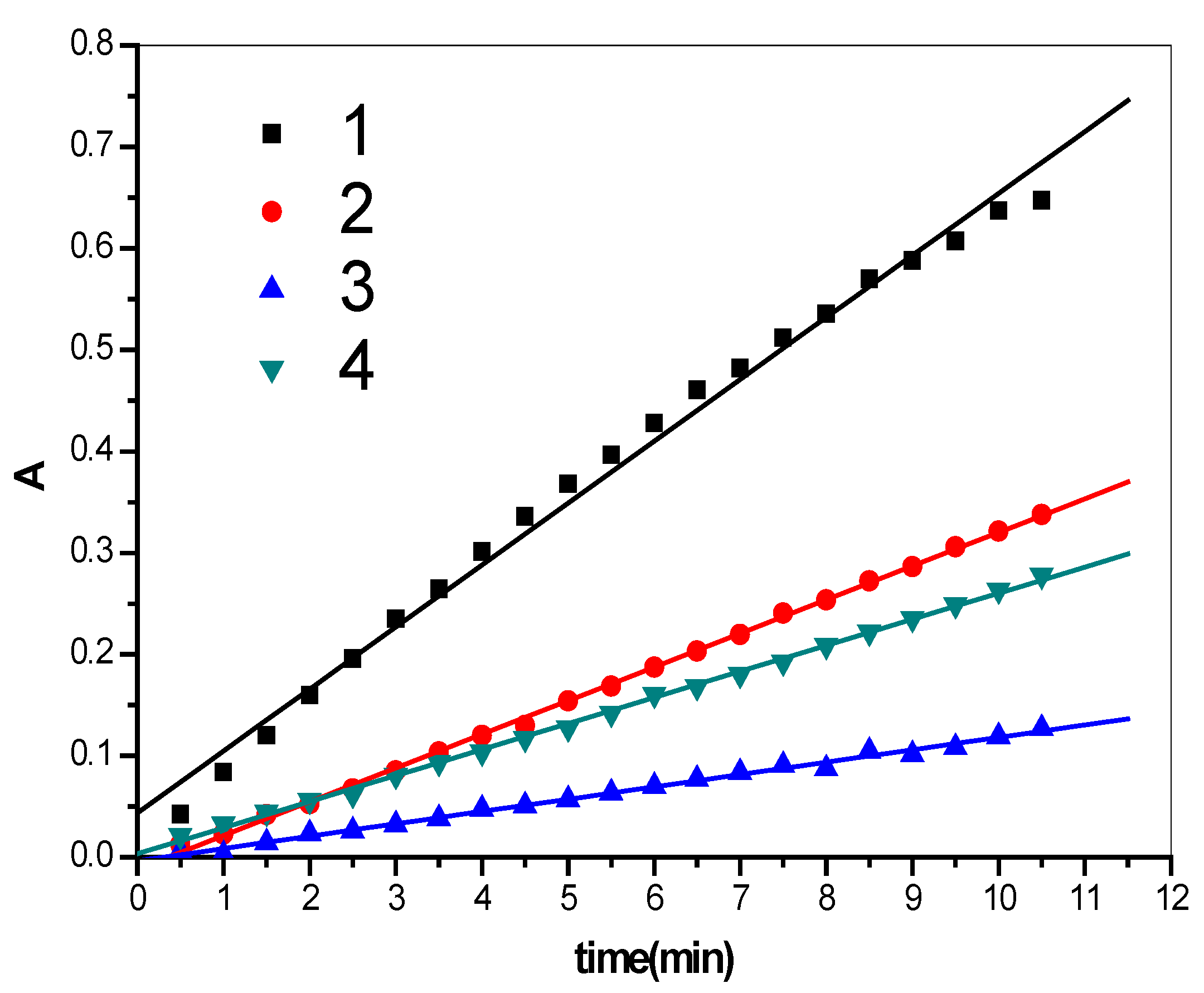
| Number | Regression equation | r | SD |
|---|---|---|---|
| Line 1 | y = 0.0437 + 0.0611x | 0.9959 | 0.0171 |
| Line 2 | y = −0.0117 + 0.0332x | 0.9996 | 0.0032 |
| Line 3 | y = −0.0036 + 0.0122x | 0.9968 | 0.0031 |
| Line 4 | y = 0.0035 + 0.0257x | 0.9993 | 0.0032 |
2.2. Fluorescence Spectrum Experimental Results
2.2.1. Fluorescence Emission Spectra

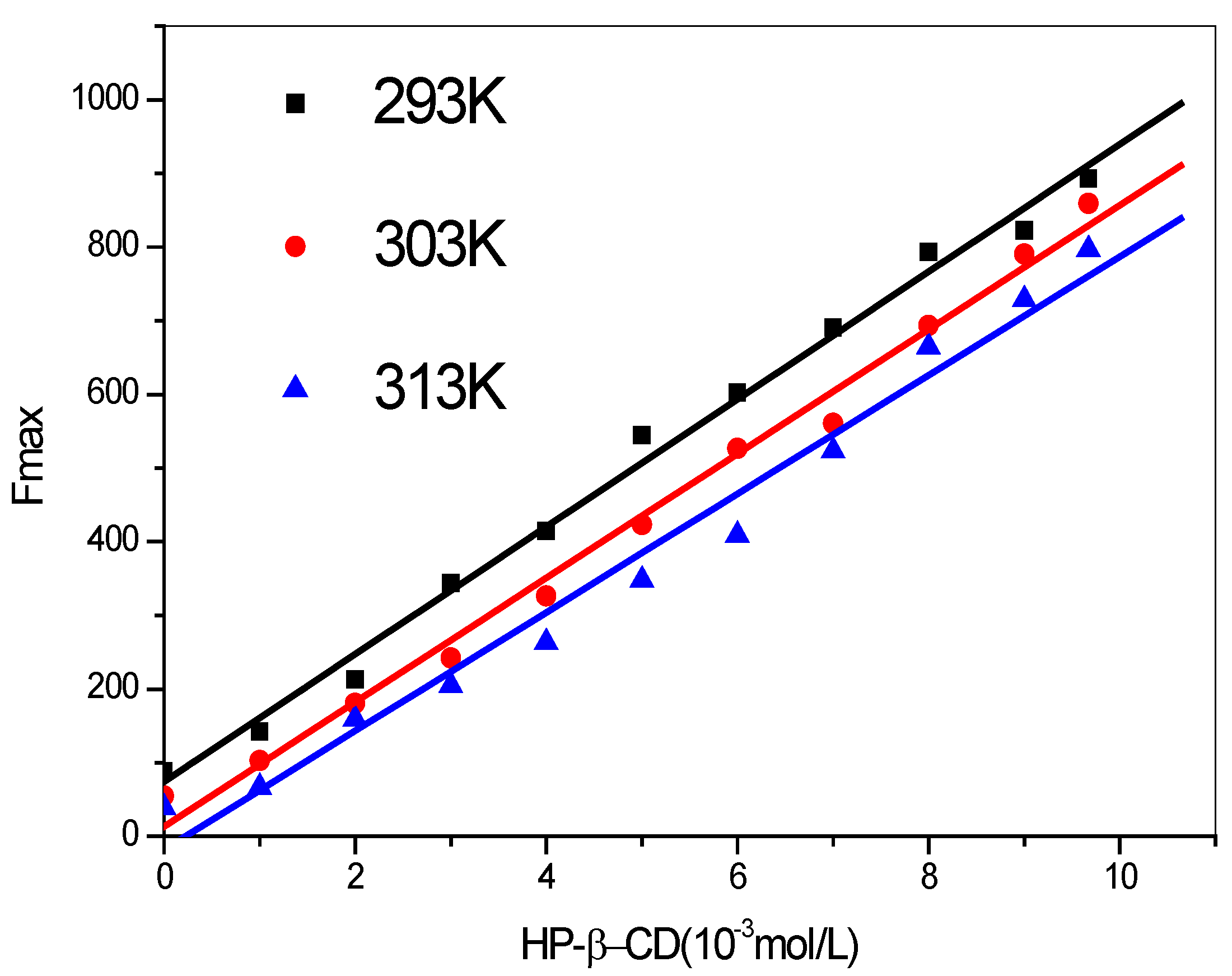
| T/K | Regression equation | r | SD |
|---|---|---|---|
| 293 | y = 73.9753 + 86.5676x | 0.9967 | 24.3745 |
| 303 | y = 13.2668 + 84.3740x | 0.9959 | 26.3180 |
| 313 | y = −17.9835 + 80.5136x | 0.9902 | 39.1664 |

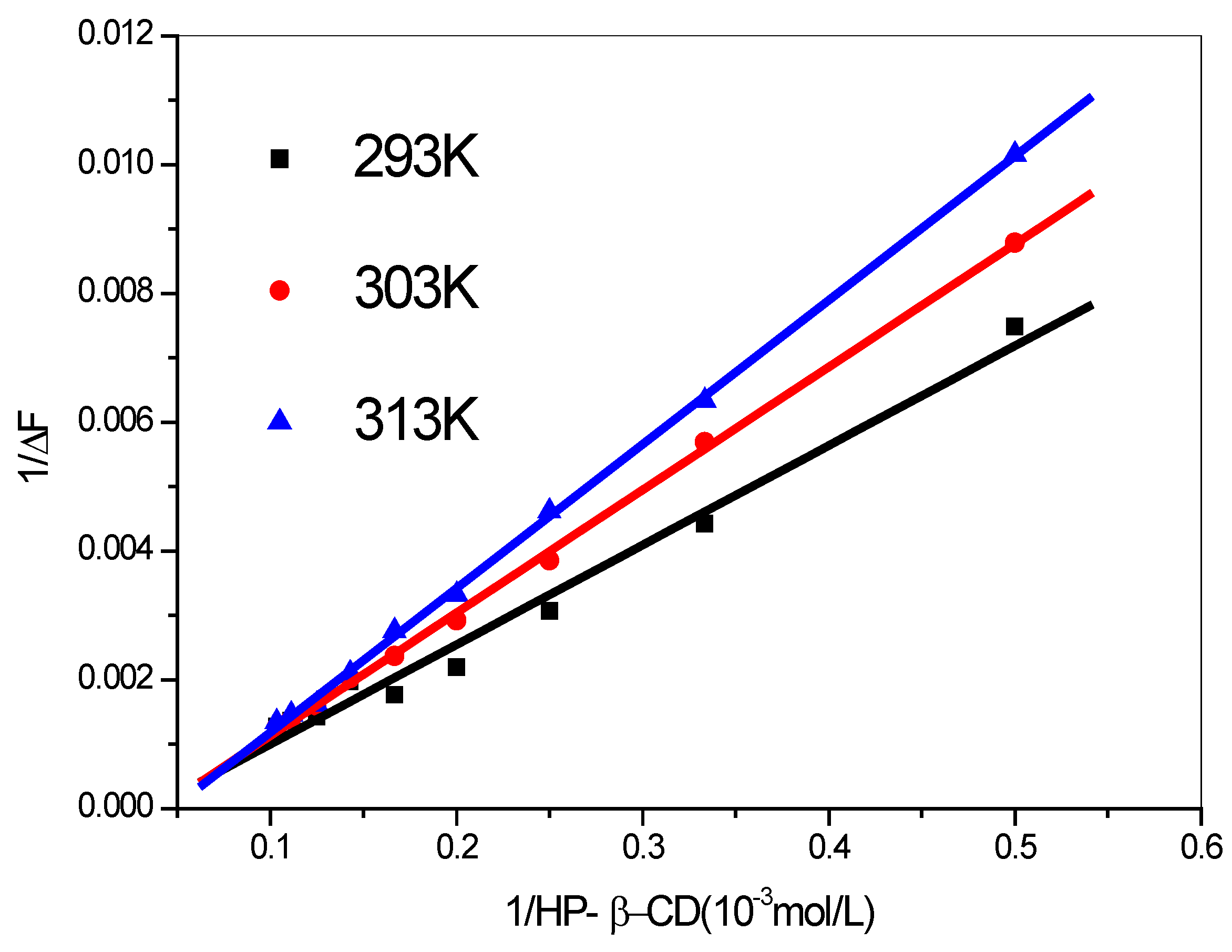
| T/K | Regression equation | r | K(M−1) | ΔG/(kJ/mol) |
|---|---|---|---|---|
| 293 | y = −5.4840 + 0.0155x | 0.9914 | 354.5 | −14.29 |
| 303 | y = −7.8066 + 0.0191x | 0.9994 | 408.7 | −15.14 |
| 313 | y = −10.60 + 0.0224x | 0.9996 | 473.6 | −16.02 |
2.2.2. Synchronous Fluorescence Spectra

2.5. Docking Analysis
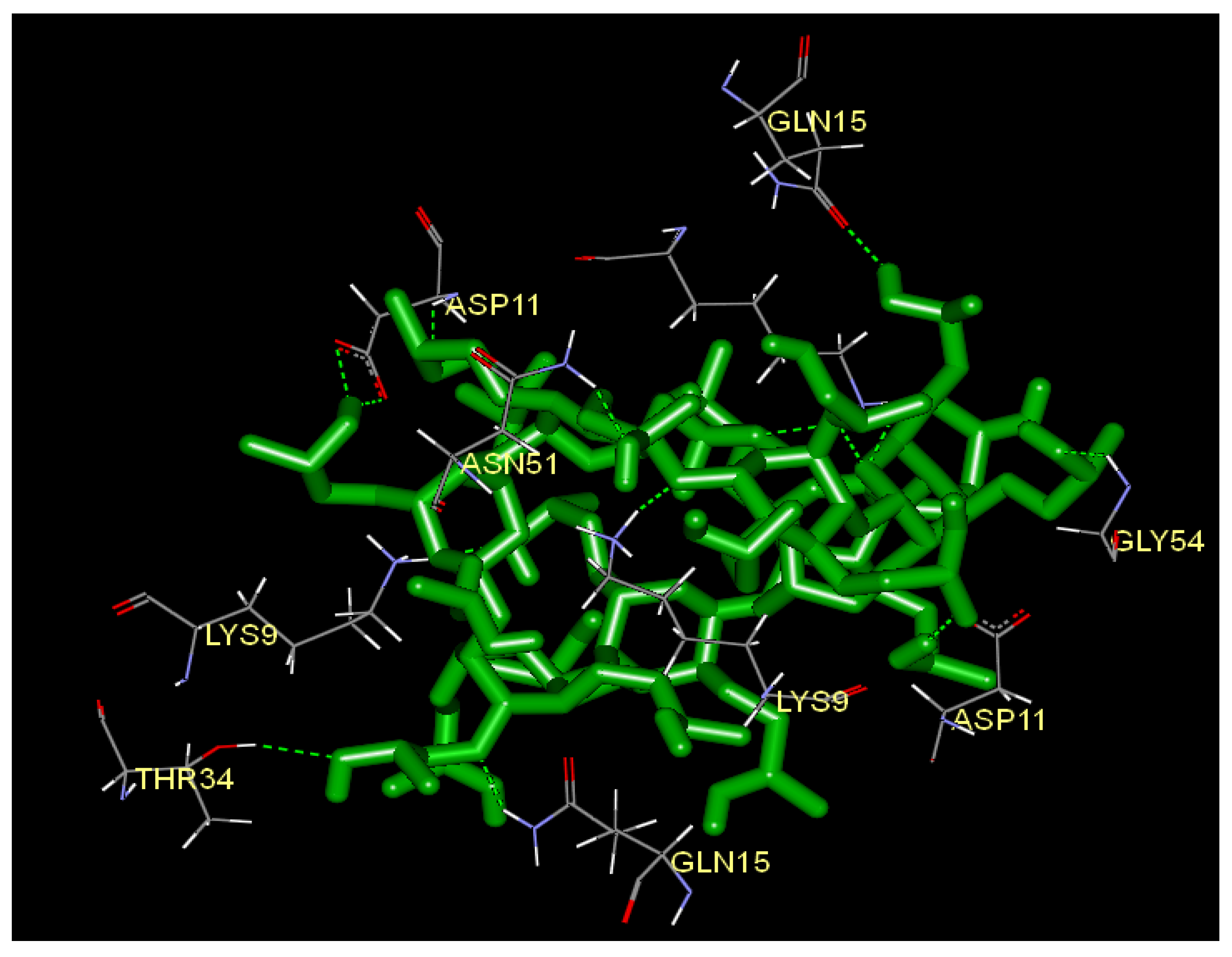
| Name | Donor Atom | Accepter Atom | Hydrogen bond distance (Å) |
|---|---|---|---|
| :HP:H-B:ASP11:OD1 | H | OD1 | 2.27962 |
| :HP:H-B:ASP11:OD2 | H | OD2 | 2.00114 |
| :HP:H-B:GLN15:OE1 | H | OE1 | 2.02632 |
| :HP:H-G:ASP11:OD1 | H | OD1 | 2.01827 |
| B:ASN51:HD21-:HP:O | HD21 | O | 1.94949 |
| B:ASP11:H-:HP:O | H | O | 2.42934 |
| B:LYS9:HZ1-:HP:O | HZ1 | O | 1.77084 |
| B:LYS9:HZ1-:HP:O | HZ1 | O | 2.35214 |
| B:LYS9:HZ2-:HP:O | HZ2 | O | 2.40027 |
| B:LYS9:HZ2-:HP:O | HZ2 | O | 1.72237 |
| B:LYS9:HZ3-:HP:O | HZ3 | O | 1.47502 |
| G:GLN15:HE22-:HP:O | HE22 | O | 1.8719 |
| G:GLY54:H-:HP:O | H | O | 2.16128 |
| G:LYS9:HZ1-:HP:O | HZ1 | O | 1.34714 |
| O:LYS9:HZ3-:HP:O | HZ3 | O | 2.07259 |
| O:THR34:HG1-:HP:O | HG1 | O | 2.34039 |
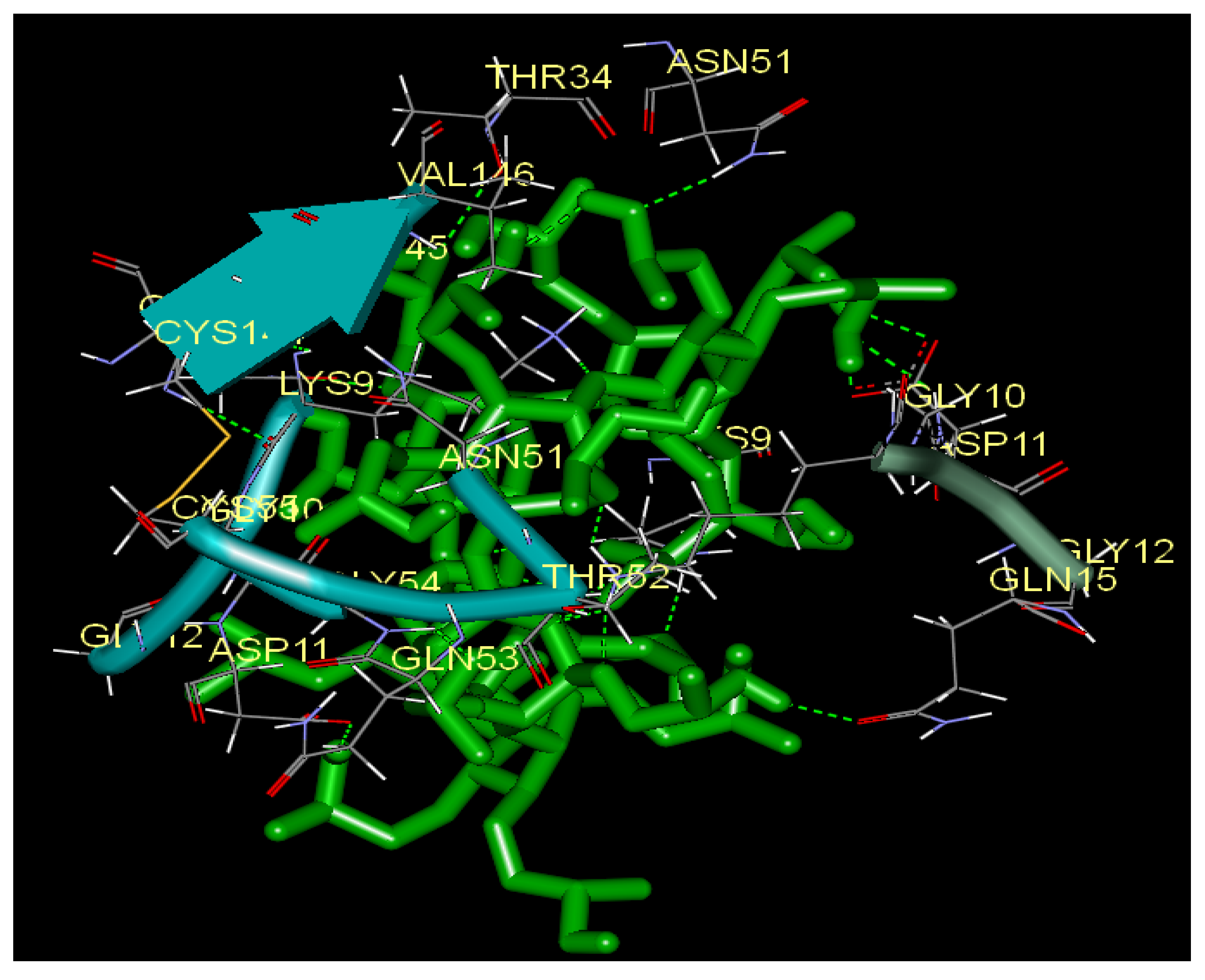
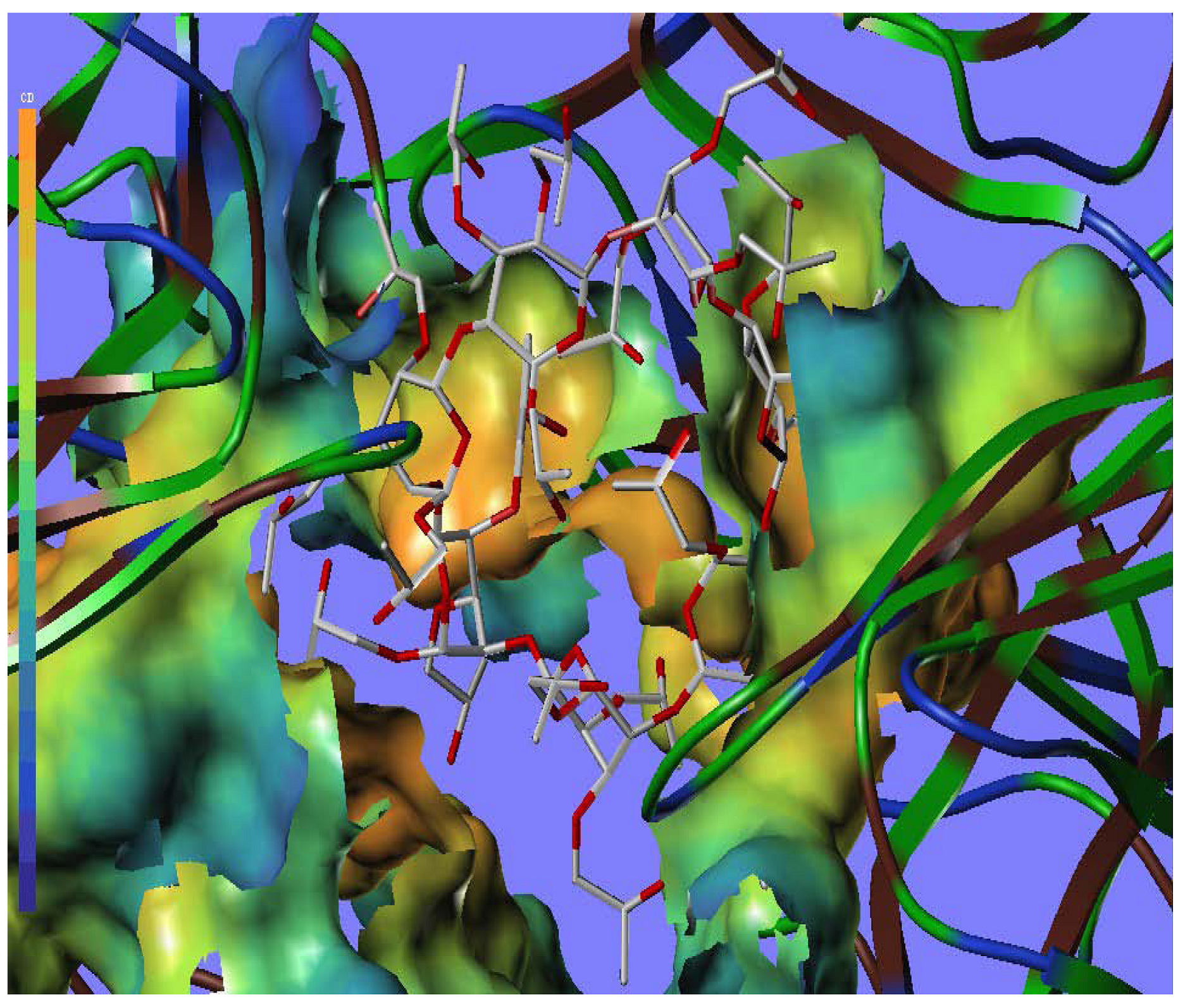
3. Experimental
3.1. Enzymatic Activity Assay
| Sample number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Tris-HCL/mL | 2.991 | 2.98 | 2.969 | 2.98 |
| Pyrogallol/mL | 0.009 | 0.009 | 0.009 | 0.009 |
| SOD/mL | -- | 0.011 | 0.022 | 0.022 |
| HP-β-CD/mL | -- | -- | -- | 0.1 |
3.2. Fluorescence Spectrometric Analysis
| Sample number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOD/mL | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| HP-β-CD/mL | 0 | 0.3 | 0.6 | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.4 | 2.7 | 2.9 |
| Tris-HCl/mL | 2.9 | 2.6 | 2.3 | 2.0 | 1.7 | 1.4 | 1.1 | 0.8 | 0.5 | 0.2 | 0 |
3.3. Docking Study
4. Conclusions
Acknowledgements
References and Notes
- Yang, B.; Lin, J.; Chen, Y.; Liu, Y. Artemether/hydroxypropyl-β-cyclodextrin host-guest system: Characterization, phase-solubility and inclusion mode. Bioorgan. Med. Chem. 2009, 17, 6311–6317. [Google Scholar]
- Cramer, F.; Hettler, H. Inclusion compounds of cyclodextrins. Naturwissenschaften 1967, 54, 625–632. [Google Scholar]
- Tanford, C. The Hydrophobic Effect. Formation of Micelles and Biological Membranes, 2nd ed; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar]
- Castronuovo, G.; Niccoli, M. Thermodynamics of inclusioncomplexes of natural and modified cyclodextrins with propranolol in aqueous solution at 298 K. Bioorgan. Med. Chem. 2006, 14, 3883–3887. [Google Scholar]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar]
- Banerjee, A.; Sengupta, B.; Chaudhuri, S.; Basu, K.; Sengupta, P.K. Encapsulation of Prodan in beta-cyclodextrin environments: A critical study via electronic spectroscopy and molecular mechanics. J. Mol. Struct. 2006, 794, 181–184. [Google Scholar]
- Chen, W.; Chang, C.-E.; Gilson, M.K. Calculation of cyclodextrin binding affinities: Energy, entropy, and implications for drug design. J. Biophys. 2004, 87, 3035–3049. [Google Scholar] [CrossRef]
- Wintgens, V.; Amiel, C. New 4-amino-N-alkylphthalimides as fluorescence probes for β-cyclodextrin inclusion complexes and hydrophobic microdomains of amphiphilic systems. J. Photochem. Photobiol. A Chem. 2004, 168, 217–226. [Google Scholar]
- Ficarra, R.; Tommasini, S.; Raneri, D. Study of flavonoids/beta-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Beyer, W.; Imlay, J.; Fridovich, I. Superoxide dismutases. Prog. Nucleic Acid Res. Mol. Biol. 1991, 40, 221–253. [Google Scholar]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Oxidative stress, antioxidants and diabetes: A review. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar]
- Ali, M.B.; Yu, K.W.; Hahn, E.J. Induction in the antioxidative systems and lipid peroxidation in suspension culture roots of Panax ginseng induced by oxygen in bioreactors. Plant Sci. 2005, 169, 833–841. [Google Scholar] [CrossRef]
- Riley, D.P. Functional mimics of superoxide dismutase enzymes as therapeutic agents. Chem. Rev. 1999, 99, 2573–2588. [Google Scholar]
- Miller, A.F. In vivo production of active nickel superoxide dismutase from Prochlorococcus marinus MIT9313 is dependent on its cognate peptidase. Curr. Opin. Chem. Biol. 2004, 8, 162–168. [Google Scholar] [CrossRef]
- Smith, C.D.; Carson, M.; van der Woerd, M. Crystal structure of peroxynitrite-modified bovine CU, ZN superoxide dismutase. Arch. Biochem. Biophys. 1992, 299, 350–355. [Google Scholar]
- Zhang, N.N.; He, Y.X.; Li, W.F. Crystal structures of holo and Cu-deficient Cu/Zn-SOD from the silkworm Bombyx mori and the implications in amyotrophic lateral sclerosis. Proteins 2010, 78, 1999–2004. [Google Scholar]
- Banci, L.; Benedetto, M.; Bertini, I. The solution structures of reduced monomeric superoxide dismutase, NMR, 36 structures. Biochemistry 1998, 37, 11780–11791. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.J.; Wang, W.Y. Inclusion complex of volatile oil from Lignum Dalbergiae Odoriferae with hydroxypropyl-β-cyclodextrin. Chin. J. Herb. Med. 2006, 37, 700–703. [Google Scholar]
- Xu, Y.H.; Li, X.P.; Fu, B.H.; Pu, C.H. Preparation of inclusion complex of hydroxypropyl-β- cyclodextrin and paeonol. Chin. J. Pharm. 2009, 40, 822–828. [Google Scholar]
- Dai, Y.J.; Zhang, W.W.; Zhu, L.Y. Study on inclusion complex of actarit with hydroxypropyl-β- cyclodextrin. Chin. J. New Drugs 2008, 17, 1695–1699. [Google Scholar]
- Xu, Y.J.; Zhao, Y.J.; Hu, H. Study on the activity of superoxide dismutase based on the rection of pyrogallol autoxodation. Chin. J. Southwest Univ. Natl. Sci. Ed. 2006, 32, 1207–1212. [Google Scholar]
- Miao, J.B.; Zhou, T.; Tang, K.W.; Song, L.T. A fluorimeric study of inclusion behavior between β-cyclodextrin derivatives and flurbiprofen. Chin. J. Spectrosc. Lab. 2010, 27, 1532–1536. [Google Scholar]
- Chaudhuri, S.; Chakraborty, S.; Sengupta, P.K. Encapsulation of serotonin in β-cyclodextrin nano-cavities: Fluorescence spectroscopic and molecular modeling studies. J. Mol. Struct. 2010, 975, 160–165. [Google Scholar]
- Guo, X.L.; Yang, Y.; Zhao, G.Y. Study on inclusion interactions of piroxicam with cyclodextrin derivatives. Spectrochim. Acta A 2003, 59, 3379–3386. [Google Scholar]
- Sklar, L.A.; Hudson, B.S.; Simoni, R.D. Conjugated polyene fatty acids as fluorescent probes: Binding to bovine serum albumin. Biochemistry 1977, 16, 5100–5108. [Google Scholar]
- Sample Availability: Commercially available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, Y.-Z.; Huang, Z.-H.; Song, F.-J. Enzymatic Activity Enhancement of Non-Covalent Modified Superoxide Dismutase and Molecular Docking Analysis. Molecules 2012, 17, 3945-3956. https://doi.org/10.3390/molecules17043945
Qiu Y-Z, Huang Z-H, Song F-J. Enzymatic Activity Enhancement of Non-Covalent Modified Superoxide Dismutase and Molecular Docking Analysis. Molecules. 2012; 17(4):3945-3956. https://doi.org/10.3390/molecules17043945
Chicago/Turabian StyleQiu, Yan-Zi, Zong-Hua Huang, and Fa-Jun Song. 2012. "Enzymatic Activity Enhancement of Non-Covalent Modified Superoxide Dismutase and Molecular Docking Analysis" Molecules 17, no. 4: 3945-3956. https://doi.org/10.3390/molecules17043945




