In Vitro Antimicrobial and Antioxidant Activities of Ethanolic Extract of Lyophilized Mycelium of Pleurotus ostreatus PQMZ91109
Abstract
:1. Introduction
2. Results and Discussion
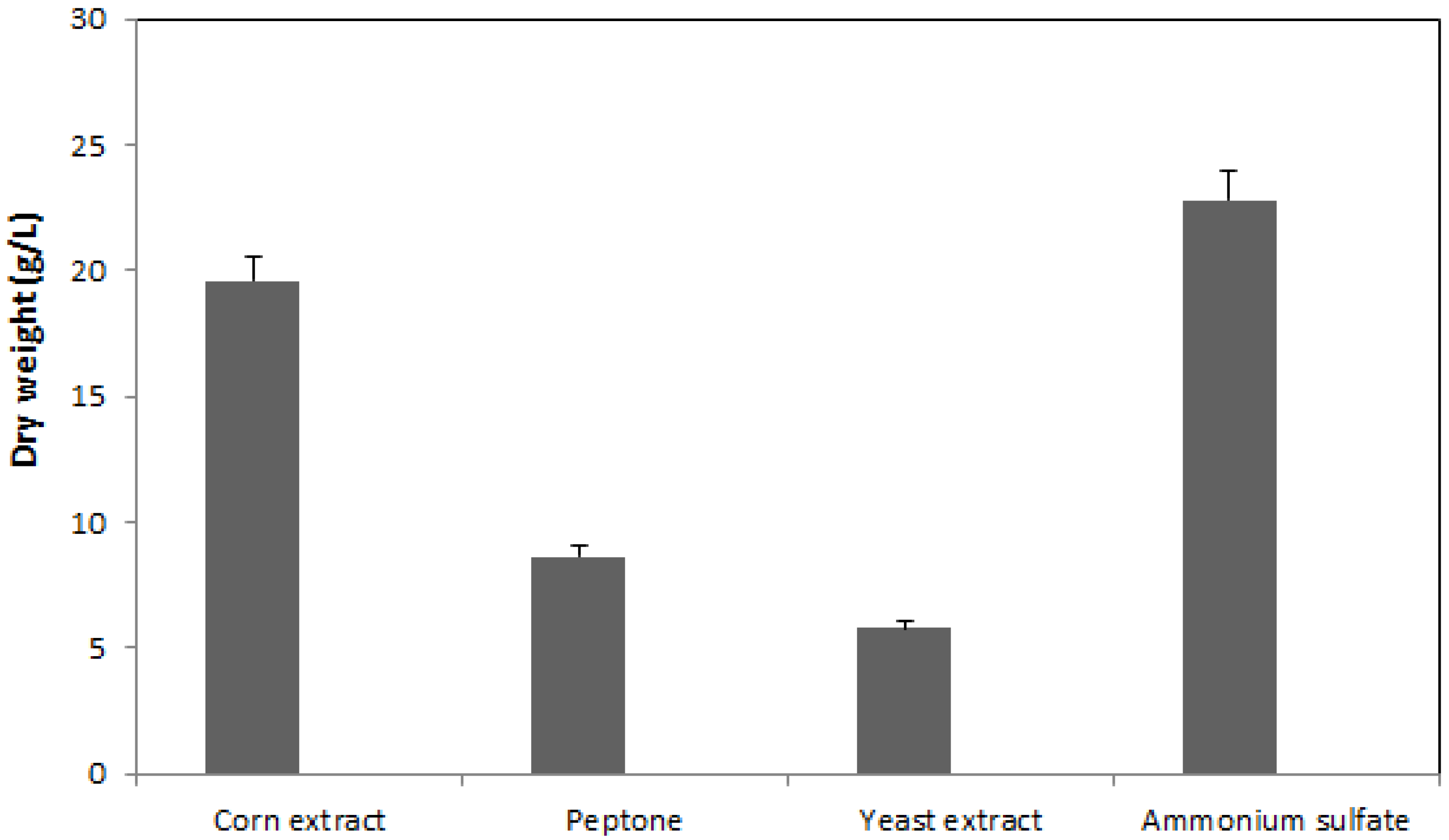
2.1. Effect of Nitrogen Source on Mycelia Growth
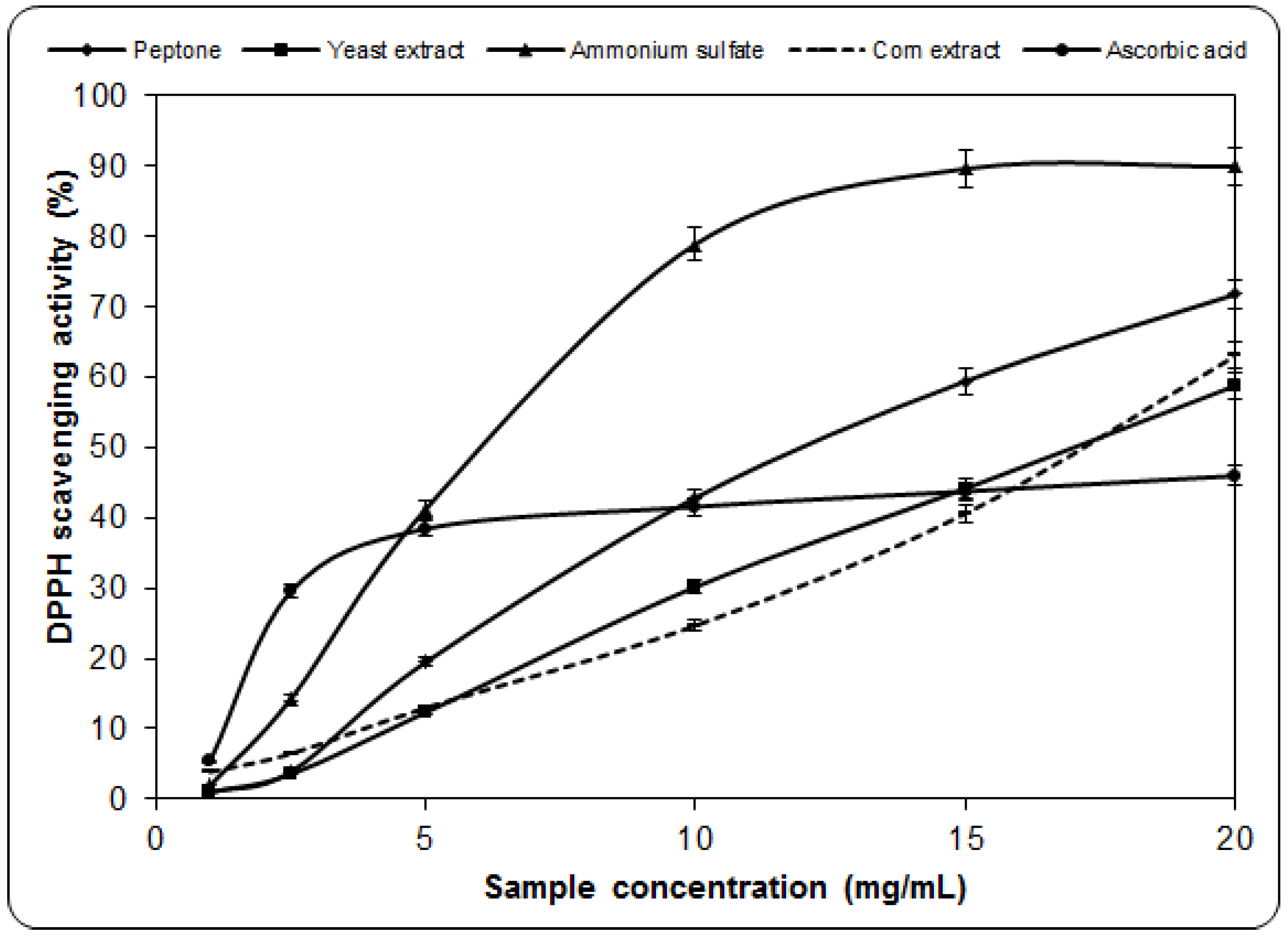
2.2. Scavenging Effect on DPPH
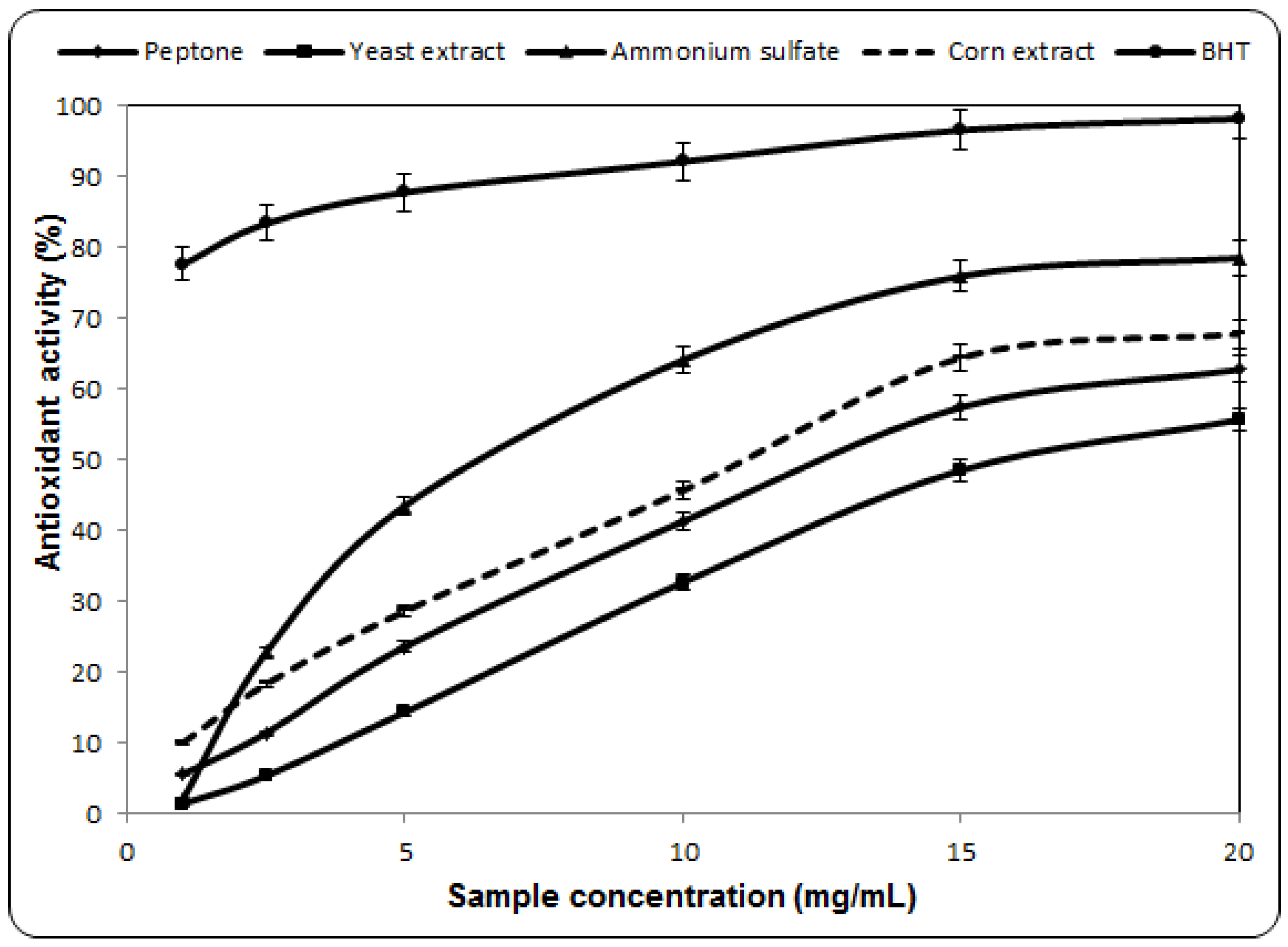
2.3. Antioxidant Activity Against β-Carotene-Linoleic Acid
2.4. Determination of Reducing Power

2.5. Scavenging Effect on Hydroxyl Radicals
| EC50 (mg/mL) | ||||
|---|---|---|---|---|
| Peptone | Yeast extract | Ammonium sulfate | Corn extract | |
| Hydroxyl scavenging activity | 8.81 ± 0.06 | 14.47 ± 0.11 | 6.54 ± 0.04 | 8.54 ± 0.02 |
| Superoxide radical scavenging activity | 2.81 ± 0.18 | 4.71 ± 0.2 | 1.27 ± 0.86 | 1.65 ± 0.61 |
| Nitric oxide scavenging activity | 1.34 ± 0.4 | 3.78 ± 0.59 | 0.77 ± 0.47 | 1.22 ± 0.85 |
| Bioactive Compounds | ||||
| Ascorbic acid (mg/100 g) | 19.34 ± 0.1 | 17.8 ± 0.05 | 20 ± 0.07 | 21 ± 0.02 |
| Total free phenolics (mg gallic acid/100 g) | 80 ± 0.12 | 66 ± 0.43 | 83 ± 0.1 | 71 ± 0.51 |
| Flavonoids (mg quercetin/100 g) | 450 ± 0.79 | 387 ± 0.41 | 531 ± 0.54 | 477 ± 0.3 |
| Lycopene (mg/100 g) | - | 0.048 ± 0.02 | 0.27 ± 0.02 | 0.32 ± 0.06 |
| β-carotene (mg/100 g) | - | - | 0.32 ± 0.12 | 0.44 ± 0.1 |
| α-tocopherol (mg/100 g) | 1.57 ± 0.58 | 1.8 ± 0.22 | 18.76 ± 1.07 | 28.59 ± 0.61 |
2.6. Scavenging Effect on Superoxide Radicals
2.7. Scavenging Activity of Nitric Oxide
2.8. Effect of Nitrogen Source on Antimicrobial Activity
| Nitrogen source | MIC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Escherichia coli CBAB 2 | Bacillus cereus CMGB 215 | Listeria innocua CMGB 218 | Candida sp.ICCF15 | Candida albicans ATCC 20231 | Pseudomonas aeruginosa ATCC 15442 | Staphylococcus aureus ATCC 6588 | |
| Corn extract | 1.25 | 12.5 | 20 | 1.25 | 1.25 | 12.5 | 12.5 |
| Ammonium sulfate | - | 2.5 | 2.5 | 1.25 | 1.25 | 2.5 | 12.5 |
| Yeast extract | - | 2.5 | 20 | 1.25 | 1.25 | 20 | 12.5 |
| Peptone | - | 5 | 2.5 | 1.25 | 1.25 | 2.5 | 12.5 |
2.9. Antioxidant Components
3. Experimental
3.1. Chemicals
3.2. Culture and Storage Condition
3.3. Media Preparation and Fermentation Condition
3.4. Preparation of Mushroom Extract
3.5. Antimicrobial Activity
Determination of Minimum Inhibitory Concentration (MIC)
3.6. Determination of Antioxidant Activities
3.6.1. 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Activity of Freeze-Dried Mushroom Extracts
3.6.2. Antioxidant Activity by β-Carotene-Linoleic Acid
3.6.3. Reducing Power of Freeze-Dried Extracts
3.6.4. Superoxide Radical Scavenging Activity of Freeze-Dried Extracts
3.6.5. Hydroxyl Radical Scavenging of Freeze-Dried Extracts
3.6.6. Nitric Oxide Scavenging of Freeze-Dried Extracts
3.7. Determination of Antioxidant Component
3.7.1. Determination of Total Phenolic Content
3.7.2. Determination of Total Flavonoids
3.7.3. Determination of β-Carotene and Lycopene
3.7.4. Determination of α-Tocopherol
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Iwalokun, B.A.; Usen, U.A.; Otunba, A.A.; Olukoya, D.K. Comparative phytochemical evaluation, antimicrobial and antioxidant properties of Pleurotus ostreatus. Afr. J. Biotechnol. 2007, 6, 1732–1739. [Google Scholar]
- Lindequist, U.; Niedermeyer, T.H.J.; Julich, W. The pharmacological potentials of mushrooms. Evid. Based Complement. Alternat. Med. 2005, 2, 285–299. [Google Scholar]
- Smith, J.E.; Rowan, N.J.; Sullivan, R. Medicinal mushrooms: A rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotechnol. Lett. 2002, 24, 1839–1845. [Google Scholar]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007, 105, 179–186. [Google Scholar]
- Jayakumar, T.; Thomas, P.A.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar]
- Tsai, S.Y.; Tsai, H.L.; Mau, J.L. Antioxidant properties of Coprinus comatus. J. Food Biochem. 2009, 33, 368–389. [Google Scholar]
- Keyhani, J.; Keyhani, E.; Attar, F.; Hadizadeh, M. Anti-oxidative stress enzymes in Pleurotus ostreatus. In Proceedings of the II International Conference on Environmental, Industrial and Applied Microbiology (BioMicroWorld2007), University of Seville, Seville, Spain, 28 November-1 December 2007; World Scientific Publishing Co.: London, UK, 2009. [Google Scholar]
- Alam, N.; Yoon, K.N.; Cha, Y.J.; Kim, J.H.; Lee, K.R.; Lee, T.S. Appraisal of the antioxidant, phenolic compounds concentration, xanthine oxidase and tyrosinase inhibitory activities of Pleurotus salmoneostramineus. Afr. J. Agric. Res. 2011, 6, 1555–1563. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, T.S. Evaluation of the antioxidant and antityrosinase activities of three extracts from Pleurotus nebrodensis fruiting bodies. Afr. J. Biotechnol. 2011, 10, 2978–2986. [Google Scholar]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar]
- Abascal, K.; Ganora, L.; Yarnell, E. The effect of freeze-drying and its implications for botanical medicine: A review. Phytother. Res. 2005, 19, 655–660. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar]
- Petre, M.; Teodorescu, A.; Ţuluca, E.; Bejan, C.; Andronescu, A. Biotechnology of mushroom pellets producing by controlled submerged fermentation. Rom. Biotechnol. Lett. 2010, 15, 50–55. [Google Scholar]
- Sung, G.H.; Shrestha, B.; Han, S.K.; Kim, S.Y.; Sung, J.M. Growth and cultural characteristics of Cordyceps cardinalis collected from Korea. Mycobiology 2010, 38, 274–281. [Google Scholar]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar]
- Arora, D.S.; Chandra, P. Antioxidant activity of Aspergillus fumigates. ISRN Pharmacol. 2011, 1, 1–11. [Google Scholar]
- Ruhul Amin, S.M.; Sarker, N.C.; Hossain, K. Optimization of in vitro culture conditions for mycelial growth of Turkey tail mushroom (Coriolus versicolor). Bangladesh J. Mushroom 2008, 2, 63–71. [Google Scholar]
- Jayakumar, T.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res. Int. 2011, 44, 851–861. [Google Scholar]
- Elmastasa, M.; Isildaka, O.; Turkekulb, I.; Temura, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compost. Anal. 2007, 20, 337–345. [Google Scholar]
- Yeh, J.Y.; Hsieh, L.H.; Wu, K.T.; Tsai, C.F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar]
- Acharya, S.; Sahu, A.R.; Mohanta, S.R. Free radical scavenging activity of thalamus of Nymphacea stellata willd. Int. J. Pharm. Pharm. Sci. 2010, 2, 61–63. [Google Scholar]
- Sarikurkcu, C.; Tepe, B.; Semiz, D.K.; Solak, M.H. Evaluation of metal concentration and antioxidant activity of three edible mushrooms from Mugla, Turkey. Food Chem. Toxicol. 2010, 48, 1230–1233. [Google Scholar] [CrossRef]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar]
- Srinivasan, K.; Jagadish, L.K.; Shenbhagaraman, R.; Muthumary, J. Antioxidant activity of endophytic fungus Phyllosticta sp. isolated from Guazuma tomentosa. J. Phytol. 2010, 2, 37–41. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Shim, M.J.; Lee, M.W.; Lee, U.Y.; Lee, T.S. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology 2010, 38, 295–301. [Google Scholar]
- Yoon, K.N.; Alam, N.; Lee, J.S.; Lee, K.R.; Lee, T.S. Detection of phenolic compounds concentration and evaluation of antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus edodes. World Appl. Sci. J. 2011, 12, 1851–1859. [Google Scholar]
- Moein, M.R.; Moein, S.; Ahmadizadeh, S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules 2008, 13, 2804–2813. [Google Scholar]
- Vidović, S.S.; Mujić, I.O.; Zeković, Z.P.; Lepojević, Ž.D.; Tumbas, V.T.; Muji, A.I. Antioxidant properties of selected Boletus mushrooms. Food Biophys. 2010, 5, 49–58. [Google Scholar] [CrossRef]
- Luo, A.; Fan, Y.; Luo, A. In vitro free radicals scavenging activities of polysaccharide from Polygonum Multiflorum Thunb. J. Med. Plants Res. 2011, 5, 966–972. [Google Scholar]
- Pal, J.; Ganguly, S.; Tahsin, K.S.; Acharya, K. In vitro free radical scavenging activity of wild edible mushroom Pleurotus squarrosulus (Mont.) Singer. Indian J. Exp. Biol. 2010, 47, 1210–1218. [Google Scholar]
- Stief, T.W. The physiology and pharmacology of singlet oxygen. Med. Hypotheses 2003, 60, 567–572. [Google Scholar]
- Jiao, Z.; Liu, J.; Wang, S. Antioxidant activities of total pigment extract from blackberries. Food Technol. Biotechnol. 2005, 43, 97–102. [Google Scholar]
- Lee, J.C.; Kim, H.R.; Kim, J.; Jang, Y.S. Antioxidant activity of ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food Chem. 2002, 50, 6490–6496. [Google Scholar]
- Kiran, B.; Raveesha, K.A. In vitro evaluation of antioxidant potentiality of seeds of Psoralea corylifolia L. World Appl. Sci. J. 2010, 8, 985–990. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar]
- Vijayavel, K.; Martinez, J.A. In vitro antioxidant and antimicrobial activities of two hawaiian marine limu: Ulva fasciata (Chlorophyta) and Gracilaria salicornia (Rhodophyta). J. Med. Food 2010, 13, 1494–1499. [Google Scholar]
- Baskar, R.; Lavanya, R.; Mayilvizhi, S.; Rajasekaran, P. Free radical scavenging activity of antitumor polysaccharide fractions isolated from Ganoderma lucidum (Fr.) P. Karst. Nat. Prod. Rad. 2008, 7, 320–325. [Google Scholar]
- Onasanwo, S.A.; Singh, N.; Olaleye, S.B.; Mishra, V.; Palit, G. Anti-ulcer and antioxidant activities of Hedranthera barteri (Hook F.) with possible involvement of H+, K+ ATPase inhibitory activity. Indian J. Med. Res. 2010, 132, 442–449. [Google Scholar]
- Ameer, A.; AL-Laith, A. Antioxidant components and antioxidant/antiradical activities of desert truffle (Tirmania nivea) from various Middle Eastern origins. J. Food Compost. Anal. 2010, 23, 15–22. [Google Scholar]
- Ameri, A.; Vaidya, J.G.; Deokule, S.S. In vitro evaluation of anti-staphylococcal activity of Ganoderma lucidum, Ganoderma praelongum and Ganoderma resinaceum from Pune, India. Afr. J. Microb. Res. 2011, 5, 328–333. [Google Scholar]
- Panthi, M.P.; Chaudhury, R.P. Antibacterial activity of some selected folklore medicinal plants from West Nepal. Sci. World 2006, 4, 16–21. [Google Scholar]
- Vinayaka, K.S.; Swarnalatha, S.P.; Preethi, H.R.; Surabhi, K.S.; Prashith Kekuda, T.R.; Sudharshan, S.J. Studies on in vitro antioxidant, antibacterial and insecticidal activity of methanolic extract of Abrus pulchellus Wall (Fabaceae). Afr. J. Basic Appl. Sci. 2009, 1, 110–116. [Google Scholar]
- Mattila, P.; Vaananen, P.S.; Kongo, K.; Aro, H.; Jalava, T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar]
- Barros, L.; Ferreira, M.J.; Queiro's, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, b-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Mau, J.L.; Lin, H.C.; Chen, C.C. Antioxidant properties of several medicinal mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077. [Google Scholar]
- Wu, X.J.; Hansen, C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J. Food Sci. 2010, 73, 1–8. [Google Scholar]
- Lee, T.T.; Huang, C.C.; Shieh, X.H.; Chen, C.L.; Chen, L.J.; Yu, B.I. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. and its immunostimulant capacity in vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5–9. [Google Scholar]
- Kontush, A.; Finckh, B.; Karten, B.; Kohlschütter, A.; Beisiegel, U. Antioxidant and prooxidant activity of alpha-tocopherol in human plasma and low density lipoprotein. J. Lipid Res. 1996, 37, 1436–1448. [Google Scholar]
- Builders, P.F.; Ezeobi, C.R.; Tarfa, F.D.; Builders, M.I. Assessment of the intrinsic and stability properties of the freeze-dried and formulated extract of Hibiscus sabdariffa Linn. (Malvaceae). Afr. J. Pharm. Pharmacol. 2010, 4, 304–313. [Google Scholar]
- Knežević, S.V.; Blažeković, B.; Štefan, M.B.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Vaz, J.A.; Ferreira, I.C.F.R.; Baptista, P.; Estevinho, L.M. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur. Food Res.Technol. 2007, 225, 151–156. [Google Scholar]
- Altaf, S.A.; Umar, D.M.; Muhammad, M.S. Production of xylanase enzyme by Pleurotus eryngii and Flamulina velutipes grown on different carbon sources under submerged fermentation. World Appl. Sci. J. 2010, 8, 47–49. [Google Scholar]
- Naraian, R.; Naveen, K.A.; Garg, S.K. Improved submerged fermentation of corn cob with mechanically broken oil seed cakes and decolorisation of textile dyes by enzyme extract of Pleurotus florida PF05. Res. Environ. Life Sci. 2009, 2, 83–90. [Google Scholar]
- Oboh, I.E.; Akerele, J.O.; Obasuyi, O. Antimicrobial activity of the ethanol extract of the aerial parts of Sida acuta burm.f. (malvaceae). Trop. J. Pharm. Res. 2007, 6, 809–813. [Google Scholar]
- Sreenivasan, S.; Darah, I.; Mohd, M.J.N.K. Free radical scavenging activity and total phenolic compounds of Gracilaria changii. Int. J. Eng. Sci. 2007, 1, 115–117. [Google Scholar]
- Sheng, Z.W.; Ma, W.H.; Gao, J.H.; Bi, Y.; Zhang, W.M.; Dou, H.T.; Jin, Z.Q. Antioxidant properties of banana flower of two cultivars in China using 2,2-diphenyl-1-picrylhydrazyl (DPPH,) reducing power, 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulphonate (ABTS) and inhibition of lipid peroxidation Assays. Afr. J. Biotechnol. 2011, 10, 4470–4477. [Google Scholar]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2007, 15, 627–634. [Google Scholar]
- Zengin, G.; Aktumsek, A.; Guler, G.O.; Cakmak, Y.S.; Yildiztugay, E. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec. Nat. Prod. 2011, 5, 123–132. [Google Scholar]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Chou, H.J.; Kuo, J.T.; Lin, E.S. Comparative antioxidant properties of water extracts from different parts of Beefsteak plant (Perilla frutescens). J. Food Drug Anal. 2009, 17, 489–496. [Google Scholar]
- Lin, E.S.; Li, C.C. Evaluation of superoxide radical scavenging capacity and reducing power of areca flower extracts. J. Med. Plants Res. 2010, 4, 975–981. [Google Scholar]
- Varshneya, C.; Varshneya, C.; Kant, V.; Mehta, M. Total phenolic contents and free radical scavenging activities of different extracts of seabuckthorn (Hippophae rhamnoides) pomace without seeds. Int. J. Food Sci. Nutr. 2012, 63, 153–159. [Google Scholar]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar]
- Sunil, K.; Dinesh, K.M.; Kamal, S.; Nidhan, S.; Bhoodev, V. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Kivcak, B.; Akay, S. Quantitative determination of a-tocopherol in Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus by TLC-densitometry and colorimetry. Fitoterapia 2005, 76, 62–66. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vamanu, E. In Vitro Antimicrobial and Antioxidant Activities of Ethanolic Extract of Lyophilized Mycelium of Pleurotus ostreatus PQMZ91109. Molecules 2012, 17, 3653-3671. https://doi.org/10.3390/molecules17043653
Vamanu E. In Vitro Antimicrobial and Antioxidant Activities of Ethanolic Extract of Lyophilized Mycelium of Pleurotus ostreatus PQMZ91109. Molecules. 2012; 17(4):3653-3671. https://doi.org/10.3390/molecules17043653
Chicago/Turabian StyleVamanu, Emanuel. 2012. "In Vitro Antimicrobial and Antioxidant Activities of Ethanolic Extract of Lyophilized Mycelium of Pleurotus ostreatus PQMZ91109" Molecules 17, no. 4: 3653-3671. https://doi.org/10.3390/molecules17043653





