Inhibitory Effect of Astragalus Polysaccharides on Lipopolysaccharide-Induced TNF-a and IL-1β Production in THP-1 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of APS on THP-1 Cell Viability
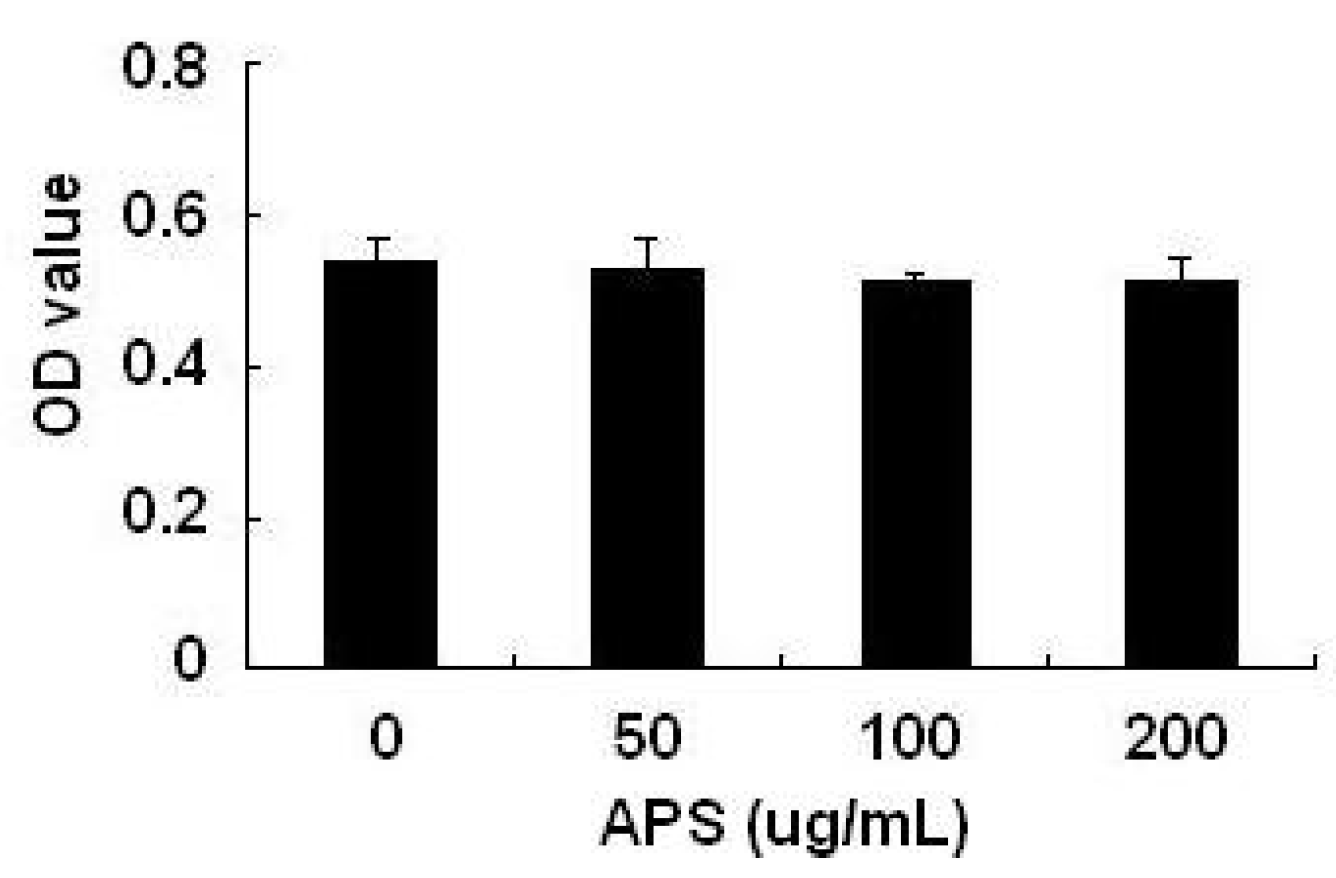
2.2. Effect of APS on LPS-Induced Cytokine Expression in THP-1 Cells


2.3. Effect of APS on NF-κB Activation in LPS-Stimulated THP-1 Cells

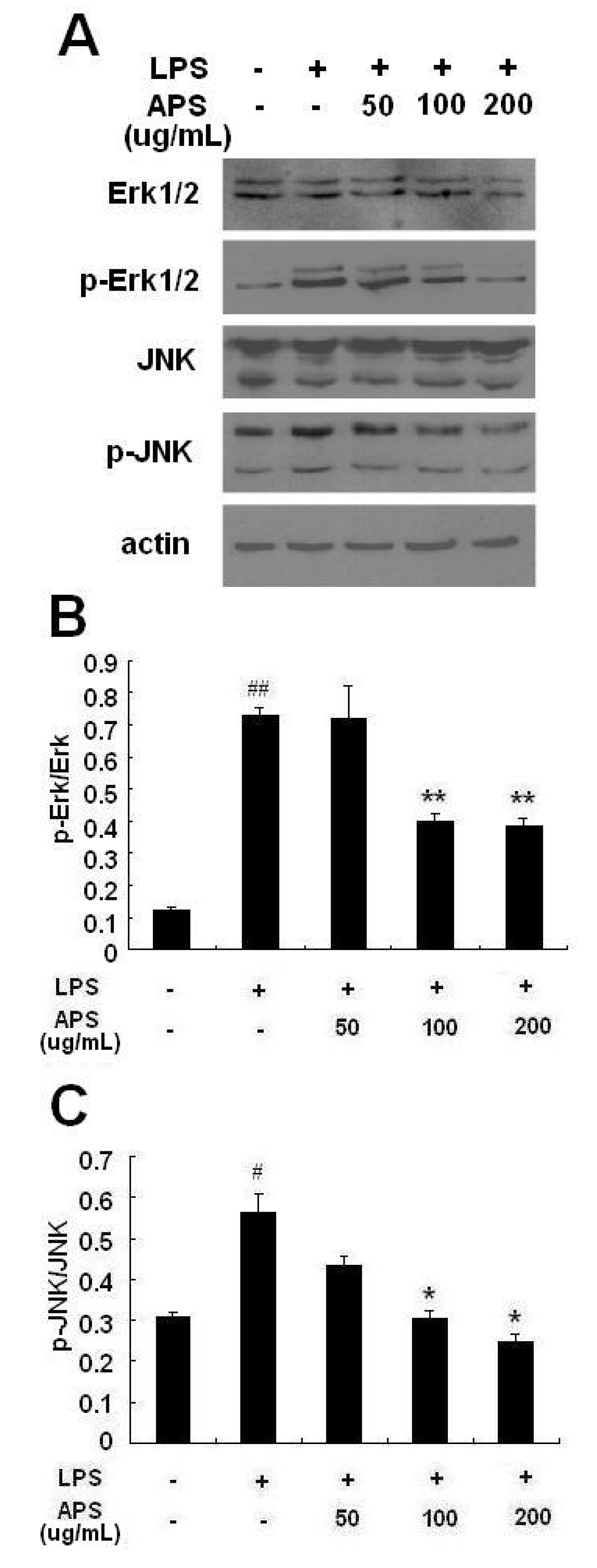
2.4. Effect of APS on Phosphorylation of ERK and JNK in LPS-Stimulated THP-1 Cells
2.5. Discussion
3. Experimental
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. MTT Assay for THP-1 Cells Viability
3.4. Macrophage Stimulation
3.5. Real-Time PCR
3.6. Cytokine Assays
3.7. Western Blot
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Anogeianaki, A.; Angelucci, D.; Cianchetti, E.; D'Alessandro, M.; Maccauro, G.; Saggini, A.; Salini, V.; Caraffa, A.; Tete, S.; Conti, F.; et al. Atherosclerosis: A classic inflammatory disease. Int. J. Immunopathol. Pharmacol. 2011, 24, 817–825. [Google Scholar]
- Mahmoudi, M.; Curzen, N.; Gallagher, P.J. Atherogenesis: The role of inflammation and infection. Histopathology 2007, 50, 535–546. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflaming metastasis. Nature 2009, 457, 36–37. [Google Scholar] [CrossRef]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef]
- Fong, K.Y.; Boey, M.L.; Koh, W.H.; Feng, P.H. Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: Correlation with bony erosions. Clin. Exp. Rheumatol. 1994, 12, 55–58. [Google Scholar]
- Duffield, J.S. The inflammatory macrophage: A story of Jekyll and Hyde. Clin. Sci. (Lond.) 2003, 104, 27–38. [Google Scholar]
- Jakobsson, P.J. Pain: How macrophages mediate inflammatory pain via ATP signaling. Nat. Rev. Rheumatol. 2010, 6, 679–681. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Qiu, D.; Kao, P.N. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 2003, 4, 1–18. [Google Scholar] [CrossRef]
- Mito, S.; Watanabe, K.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Sukumaran, V.; Suzuki, K.; Kodama, M.; Aizawa, Y. Curcumin ameliorates cardiac inflammation in rats with autoimmune myocarditis. Biol. Pharm. Bull. 2011, 34, 974–979. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, Y.; Hou, X.; Xu, J.; Mu, X.; Chen, W. Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol. 2011, 137, 914–920. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Hu, S.J.; Qiu, L.H.; Shan, Q.X.; Sun, J.; Xia, Q.; Bian, K. Diphasic effects of Astragalus membranaceus BUNGE (Leguminosae) on vascular tone in rat thoracic aorta. Biol. Pharm. Bull. 2005, 28, 1450–1454. [Google Scholar]
- Jiang, J.B.; Qiu, J.D.; Yang, L.H.; He, J.P.; Smith, G.W.; Li, H.Q. Therapeutic effects of astragalus polysaccharides on inflammation and synovial apoptosis in rats with adjuvant-induced arthritis. Int. J. Rheum. Dis. 2010, 13, 396–405. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, M.; Li, K.S. Astragalus mongholicus polysaccharide inhibits lipopolysaccharide-induced production of TNF-alpha and interleukin-8. World J. Gastroenterol. 2009, 15, 3676–3680. [Google Scholar]
- Shao, P.; Zhao, L.H.; Zhi, C.; Pan, J.P. Regulation on maturation and function of dendritic cells by Astragalus mongholicus polysaccharides. Int. Immunopharmacol. 2006, 6, 1161–1166. [Google Scholar] [CrossRef]
- Dang, S.S.; Zhang, X.; Jia, X.L.; Cheng, Y.A.; Song, P.; Liu, E.Q.; He, Q.; Li, Z.F. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin. Med. J. (Engl.) 2008, 121, 1010–1014. [Google Scholar]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Lu, Y.; Stinnette, T.W.; Westrick, E.; Klein, P.J.; Gehrke, M.A.; Cross, V.A.; Vlahov, I.R.; Low, P.S.; Leamon, C.P. Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res. Ther. 2011, 13, R56. [Google Scholar] [CrossRef]
- Le Loet, X.; Nordstrom, D.; Rodriguez, M.; Rubbert, A.; Sarzi-Puttini, P.; Wouters, J.M.; Woolley, J.M.; Wright, N.; Lawrence, C.; Appleton, B. Effect of anakinra on functional status in patients with active rheumatoid arthritis receiving concomitant therapy with traditional disease modifying antirheumatic drugs: Evidence from the OMEGA Trial. J. Rheumatol. 2008, 35, 1538–1544. [Google Scholar]
- Malozowski, S.; Sahlroot, J.T. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 357, 302–303. [Google Scholar] [CrossRef]
- Nam, J.; Emery, P. Aspects of TNF inhibitor therapy in rheumatoid arthritis. Mod. Rheumatol. 2010, 20, 325–330. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: A longitudinal observational study. Arthritis Res. Ther. 2010, 12, R35. [Google Scholar] [CrossRef]
- Salliot, C.; Dougados, M.; Gossec, L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: Meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 2009, 68, 25–32. [Google Scholar]
- Wallis, R.S. Tumour necrosis factor antagonists: Structure, function, and tuberculosis risks. Lancet Infect. Dis. 2008, 8, 601–611. [Google Scholar] [CrossRef]
- Desai, S.B.; Furst, D.E. Problems encountered during anti-tumour necrosis factor therapy. Best Pract. Res. Clin. Rheumatol. 2006, 20, 757–90. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar]
- Cao, W.; Bao, C.; Padalko, E.; Lowenstein, C.J. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J. Exp. Med. 2008, 205, 1491–1503. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chang, C.Y.; Lin, K.L.; Hu, C.M.; Lin, C.H.; Kang, J.J. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-kappaB signaling. J. Ethnopharmacol. 2008, 120, 264–271. [Google Scholar]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-alpha and IL-6 production in mouse macrophages by inhibiting NF-kappaB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; He, J.; Wei, M.; Yang, X.; Cao, Q.; Li, H.; Guan, S.; Deng, Y.; Pang, D.; et al. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules 2011, 16, 7634–7648. [Google Scholar] [CrossRef]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar]
- Mizukami, J.; Takaesu, G.; Akatsuka, H.; Sakurai, H.; Ninomiya-Tsuji, J.; Matsumoto, K.; Sakurai, N. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol. Cell. Biol. 2002, 22, 992–1000. [Google Scholar]
- Ninomiya-Tsuji, J.; Kishimoto, K.; Hiyama, A.; Inoue, J.; Cao, Z.; Matsumoto, K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999, 398, 252–256. [Google Scholar] [CrossRef]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, X.; Shu, J.; Xu, L.; Lu, C.; Lu, A. Inhibitory Effect of Astragalus Polysaccharides on Lipopolysaccharide-Induced TNF-a and IL-1β Production in THP-1 Cells. Molecules 2012, 17, 3155-3164. https://doi.org/10.3390/molecules17033155
He X, Shu J, Xu L, Lu C, Lu A. Inhibitory Effect of Astragalus Polysaccharides on Lipopolysaccharide-Induced TNF-a and IL-1β Production in THP-1 Cells. Molecules. 2012; 17(3):3155-3164. https://doi.org/10.3390/molecules17033155
Chicago/Turabian StyleHe, Xiaojuan, Jun Shu, Li Xu, Cheng Lu, and Aiping Lu. 2012. "Inhibitory Effect of Astragalus Polysaccharides on Lipopolysaccharide-Induced TNF-a and IL-1β Production in THP-1 Cells" Molecules 17, no. 3: 3155-3164. https://doi.org/10.3390/molecules17033155



