2.2. Total Phenolic Content and SET-Type Antioxidant Activity of the Sorbus Dry Extracts versus Phenolic Standards
In our previous work [
8] it was proved that the total phenolic content (TPC) as determined by the Folin-Ciocalteu (FC) assay is a good approximate of the total level of the main phenolic metabolites of
Sorbus tissues, including flavonoids, proanthocyanidins and caffeoylquinic acids. Thus, the FC method was chosen in the present study to screen the phenolic content of the analysed samples (
Table 2).
Table 2.
Total phenolic content and SET-type antioxidant activity of the analysed Sorbus dry extracts and fractions a.
Table 2.
Total phenolic content and SET-type antioxidant activity of the analysed Sorbus dry extracts and fractions a.
| Sample No. | Extract/ Fraction | Total phenolic content (TPC)
b | Radical-scavenging activity (RSC)
c | Reducing power
d |
|---|
| GAE (%) | DPPH EC50 (µg/mL) | TEAC (mmol Trolox®/g) | FRAP (mmol Fe2+/g) |
|---|
| 1. | ME | 21.17 ± 0.67
M | 8.93 ± 0.27
I | 1.72 ± 0.06
L | 4.43 ± 0.14
N,P |
| | DEF | 37.61 ± 0.37
H,I | 5.53 ± 0.22
E,F | 2.14 ± 0.09
I,J,K | 9.30 ± 0.38
G,H,I |
| | EAF | 54.34 ± 0.46
B,C | 3.37 ± 0.18
A,B,C | 3.22 ± 0.10
E | 12.77 ± 0.12
B,C |
| | BF | 48.71 ± 1.27
E,F | 3.52 ± 0.13
A,B,C | 3.58 ± 0.12
C,D | 10.84 ± 0.17
F |
| | WR | 9.05 ± 0.15
P | 9.96 ± 0.19
K | 0.94 ± 0.04
R | 2.58 ± 0.05
R,S |
| 2. | ME | 23.77 ± 0.30L,M | 7.16 ± 0.22
G | 1.70 ± 0.10
L | 5.04 ± 0.24
M,N |
| | DEF | 36.67 ± 0.49
H I | 5.72 ± 0.20
E,F | 2.14 ± 0.05
I,J,K | 7.58 ± 0.10
K |
| | EAF | 53.55 ± 1.13
C,D | 3.52 ± 0.13
A,B,C | 2.62 ± 0.13
H | 12.23 ± 0.07
C,D |
| | BF | 48.52 ± 0.53
E,F,G | 3.53 ± 0.16
A,B,C | 3.40 ± 0.08
D,E | 11.01 ± 0.59
E,F |
| | WR | 11.00 ± 0.12
P | 9.66 ± 0.25
K | 1.26 ± 0.05
N | 2.70 ± 0.13
R,S |
| 3. | ME | 24.61 ± 0.82
L | 7.76 ± 0.16
H | 1.79 ± 0.09
L | 5.42 ± 0.16
M |
| | DEF | 34.50 ± 0.89
I,J | 5.57 ± 0.14
E,F | 2.67 ± 0.08
G,H | 8.50 ± 0.10
J |
| | EAF | 55.16 ± 0.79
A,B,C | 3.44 ± 0.07
A,B,C | 3.98 ± 0.14
A | 13.74 ± 0.16
A |
| | BF | 53.75 ± 1.62
B,C,D | 3.17 ± 0.11
A | 3.55 ± 0.11
B,C,D | 11.47 ± 0.11
E,F |
| | WR | 10.06 ± 0.66
P | 9.84 ± 0.19
K | 1.21 ± 0.03
N | 2.77 ± 0.05
R,S |
| 4. | ME | 24.63 ± 0.22
L | 7.93 ± 0.16
H | 1.99 ± 0.04
K | 5.36 ± 0.28
M |
| | DEF | 36.87 ± 0.80
H,I | 5.39 ± 0.21
D,E | 2.71 ± 0.07
G,H | 9.34 ± 0.30
G,H |
| | EAF | 54.09 ± 0.34
B,C | 3.71 ± 0.18
B,C | 3.65 ± 0.12
B,C | 13.06 ± 0.26
B |
| | BF | 57.09 ± 0.50
A,B | 3.25 ± 0.12
A,B | 3.68 ± 0.12
B,C | 9.92 ± 0.36
G |
| | WR | 8.21 ± 0.31
P | 10.12 ± 0.21
K | 1.15 ± 0.04
N,P | 2.26 ± 0.06
S |
| 5. | ME | 30.62 ± 0.60
K | 6.60 ± 0.14
G | 2.12 ± 0.08
I,J,K | 6.20 ± 0.25
L |
| | DEF | 34.90 ± 0.27
I,J | 5.29 ± 0.18
D,E | 2.14 ± 0.07
I,J,K | 8.72 ± 0.27
H,I,J |
| | EAF | 52.37 ± 0.38
C,D | 3.70 ± 0.08
B,C | 3.72 ± 0.12
B | 12.94 ± 0.30
B |
| | BF | 48.62 ± 1.02
F,G | 3.83 ± 0.17
C | 3.33 ± 0.10
E | 11.05 ± 0.35
E,F |
| | WR | 11.45 ± 0.28
P | 9.54 ± 0.21
J,K | 1.31 ± 0.05
N | 2.98 ± 0.11
R |
| 6. | ME | 26.38 ± 0.91
L | 6.74 ± 0.13
G | 2.08 ± 0.10
J,K | 5.44 ± 0.25
M |
| | DEF | 32.10 ± 0.33
J,K | 5.70 ± 0.12
E,F | 2.60 ± 0.10
H | 8.38 ± 0.23
J |
| | EAF | 50.51 ± 0.95
D,E | 3.46 ± 0.17
A,B,C | 3.56 ± 0.13
C,D | 12.87 ± 0.17
B |
| | BF | 58.17 ± 0.76
A | 3.15 ± 0.13
A | 3.94 ± 0.15
A | 9.81 ± 0.19
G |
| | WR | 10.51 ± 0.30
P | 9.71 ± 0.22
K | 1.29 ± 0.04
N | 2.54 ± 0.15
R,S |
| 7. | ME | 24.03 ± 0.23
L,M | 6.84 ± 0.16
G | 1.81 ± 0.09
L | 5.54 ± 0.20
M |
| | DEF | 42.85 ± 0.87
G | 4.89 ± 0.14
D | 2.28 ± 0.08
I | 10.92 ± 0.11
F |
| | EAF | 53.29 ± 0.23
C,D | 3.80 ± 0.14
C | 3.44 ± 0.10
D,E | 11.42 ± 0.47
E,F |
| | BF | 39.56 ± 1.47
H | 5.18 ± 0.11
D,E | 2.96 ± 0.12
F | 8.67 ± 0.22
I,J |
| | WR | 10.38 ± 0.51
P | 9.83 ± 0.27
K | 1.03 ± 0.04
P,R | 2.92 ± 0.04
R |
| 8. | ME | 29.93 ± 0.43
K | 6.01 ± 0.23
F | 2.24 ± 0.11
I,J | 6.78 ± 0.16
L |
| | DEF | 53.13 ± 1.38
C,D | 3.67 ± 0.13
B,C | 2.97 ± 0.12
F | 11.60 ± 0.15
D,E |
| | EAF | 54.34 ± 0.32
B,C | 3.45 ± 0.16
A,B,C | 3.41 ± 0.11
D,E | 12.55 ± 0.54
B,C |
| | BF | 48.37 ± 0.51
E,F,G | 3.28 ± 0.15
A,B | 2.83 ± 0.11
G | 10.99 ± 0.09
E,F |
| | WR | 15.27 ± 0.18
N | 9.04 ± 0.26
I,J | 1.51 ± 0.08
M | 4.03 ± 0.11
P |
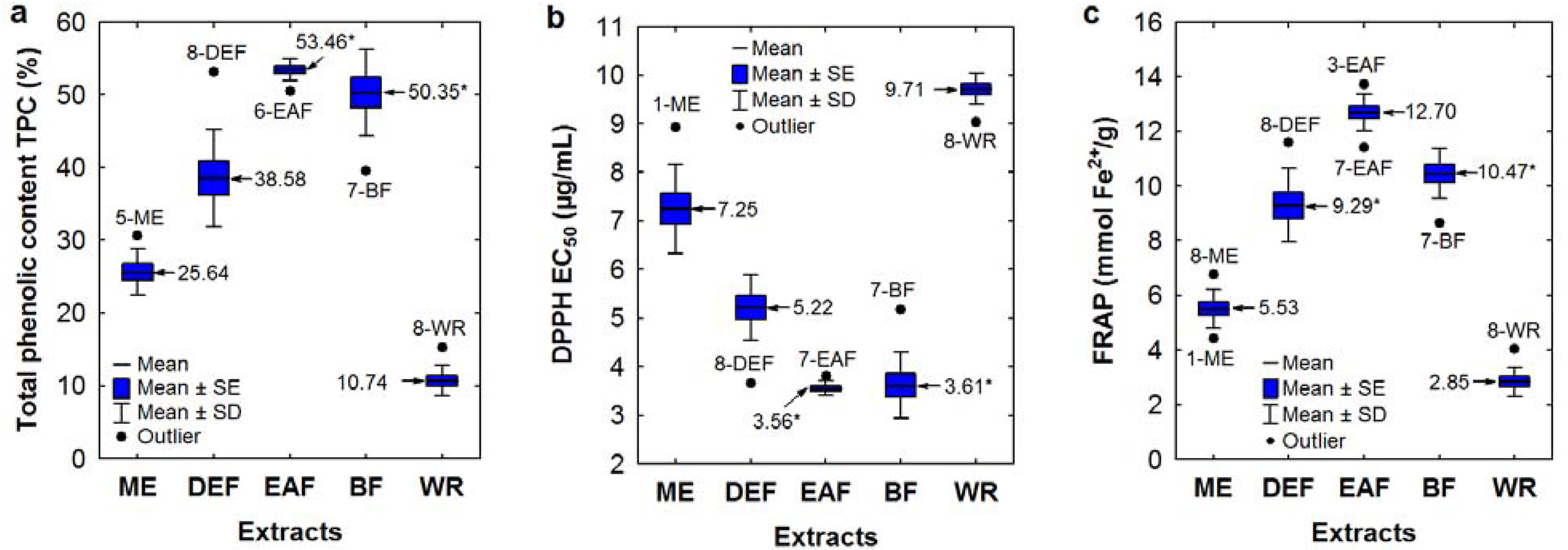
The measured TPC levels, expressed as gallic acid equivalents (GAE), were affected primarily by the extracting solvents as shown in the boxplot (
Figure 1a). The highest TPC contents were observed for EAFs and BFs (48.52–58.17% dw of the extract) with one outlier found for BF of the
S. pogonopetala leaf (39.56%), and with no significant differences between the average values for both extract groups. A similar TPC level was also noted in DEF of the
S. wilfordii leaf (53.13%). Extremely low TPC content was found for the CHEs (0.71–0.75%, results not shown), which were thus excluded from the activity and HPLC fingerprint studies.
Figure 1.
(
a–
c) Variation in total phenolic levels TPC and SET-type antioxidant activity among the
Sorbus extracts depending on the type of extraction solvent. Sample codes are given acc. to
Table 2. Mean values are given ± standard error (SE) and standard deviation (SD). Mean values marked with an asterisk are not significantlydifferent (
p < 0.01).
Figure 1.
(
a–
c) Variation in total phenolic levels TPC and SET-type antioxidant activity among the
Sorbus extracts depending on the type of extraction solvent. Sample codes are given acc. to
Table 2. Mean values are given ± standard error (SE) and standard deviation (SD). Mean values marked with an asterisk are not significantlydifferent (
p < 0.01).
The free radical scavenging activity (RSC) of the analytes was tested by two discolouration methods, such as the DPPH [
10] and ABTS (TEAC III) [
11] assays. In these methods, the antiradical capacity is expressed as the percentage decrease of the initial concentration of the DPPH radical or the initial absorbance of the ABTS
●+ solution, and further characterised by the EC
50 values. Since the results are strongly affected by the initial parameters, constant reaction conditions are crucial to maintain accuracy. The common practice to equilibrate the radical solutions to the initial absorbance of 0.700 ± 0.020 (0.030) [
7,
8,
9,
10,
12] is only partially effective, because even small differences in the initial absorbance could lead to scattered values of EC
50. Moreover, the ABTS
●+ radical cation is very unstable [
13] and the DPPH solution is sensitive to light [
15], thus both reagents could slowly deteriorate during the reaction period. On the other hand, the ratio between the initial DPPH concentration and the EC
50 value is constant [
15]. Therefore, in the present work we proposed to enhance the accuracy of the scavenging tests by the following procedure: once the initial absorbances were equilibrated, the negative controls were incubated simultaneously with the real samples to compensate possible deterioration of the radical reagents, and the calculated original values of EC
50 were normalised with the constant initial parameters (DPPH concentration of 25 μg/mL and absorbance of the ABTS
●+ solution of 0.700) by simple mathematic conversions (see
Section 3.5 and
Section 3.6). A graphical example of normalisation of the ABTS test is shown in
Figure 2.
Figure 2.
Scavenging of the ABTS radical cation by quercetin (QU)–example of normalisation of EC50 value with the absorbance of 0.700.
Figure 2.
Scavenging of the ABTS radical cation by quercetin (QU)–example of normalisation of EC50 value with the absorbance of 0.700.
The normalised EC
50 and TEAC values of the
Sorbus extracts varied from 3.15 to 10.12 μg/mL for the DPPH test, and from 0.94 to 3.98 mmol Trolox
®/g for the TEAC assay (
Table 2). High consistency of the RSC values determined by the two methods was confirmed by a statistically significant linear correlation (
r = −0.9258,
p < 0.01). The activity parameters of the dry extracts were also significantly (
p < 0.01) correlated with the TPC content. The correlation was strong for both methods, DPPH (
r = −0.9850) and TEAC (
r = 0.9361). For the DPPH tests the correlation with the TPC levels was even stronger than found previously for the
in situ methanolic liquid extracts [
8], which could be a consequence of the purification and concentration of phenolics during the preparation of dry extracts.
Similarly as observed for the FC method, variation in the RSC values for both antiradical tests was primarily caused by the differences in extraction solvents (
Figure 1b), and the highest activity was found for EAFs and BFs with no significant differences (
p < 0.01) between the average RSC values for these extract types. In the DPPH test, the EC
50 values of EAFs and BFs varied in a narrow range of 3.15–3.83 μg/mL with one outlier for BF of
S. pogonopetala leaf (5.18 μg/mL). The range of the TEAC values was slightly wider (2.83–3.98 mmol Trolox
®/g, including outliers). This activity was comparable or even higher than the RSC of the phenolic standards, such as BHA, BHT, CHA, RT, TBHQ and Trolox
® (
Table 3). Activity of CA, CFA, GA and QU was 2–3 times higher. Differences in RSC values between
Sorbus extracts and standards were more pronounced in the TEAC assay, and they could be explained by different steric accessibility of the radical sites of ABTS
●+ and DPPH radicals to small molecular standards and larger molecules [
13], such as
Sorbus phenolics including proanthocyanidins, flavonoid glycosides and caffeoylquinic acids (see
Section 2.3).
Table 3.
Antioxidant activity of the reference standards a.
Table 3.
Antioxidant activity of the reference standards a.
| Standard
b | Radical-scavenging activity (RSC)
c | Reducing power
d | LA peroxidation
e |
|---|
| DPPH EC50 (µg/mL) | TEAC (mmol Trolox®/g) | FRAP (mmol Fe2+/g) | IC50 (µg/mL) |
|---|
| CFA | 1.94 ± 0.08
A | 10.37 ± 0.17
C | 44.17 ± 0.98
A | 24.96 ± 1.34
C |
| CA | 2.17 ± 0.11
A | 9.51 ± 0.53
D | 25.37 ± 0.44
C | 69.68 ± 0.70
G |
| CHA | 4.42 ± 0.13
C | 4.13 ± 0.10
G | 18.04 ± 0.79
D | 52.47 ± 2.03
F |
| GA | 0.95 ± 0.05
D | 22.36 ± 0.63
A | 43.52 ± 1.93
A | 23.97 ± 0.98
B,C |
| QU | 1.63 ± 0.07
E | 12.41 ± 0.11
B | 36.02 ± 1.10
B | 48.51 ± 1.74
E |
| RT | 3.44 ± 0.09
F | 4.45 ± 0.15
G | 11.89 ± 0.70
F | 67.73 ± 0.34
G |
| BHA | 2.90 ± 0.14
B | 7.09 ± 0.17
E | 16.13 ± 0.83
E | 14.33 ± 0.70
A |
| BHT | 6.54 ± 0.28
G | 2.56 ± 0.08
H | 18.89 ± 0.42
D | 21.58 ± 0.95
B |
| TBHQ | 2.73 ± 0.12
B | 6.01 ± 0.24
F | 15.50 ± 0.71
E | 36.53 ± 1.04
D |
| Trolox® | 4.34 ± 0.22
C | 3.99 ± 0.10
G | 10.83 ± 0.32
F | 22.45 ± 1.10
B,C |
In the FRAP method, the antioxidant activity is determined based on the ability to reduce Fe
3+ to Fe
2+, and the results are expressed as millimolar ferrous ion equivalents per gram of the sample [
16]. The FRAP values obtained for
Sorbus extracts paralleled the TPC levels and the results of TEAC and DPPH tests (
Table 2,
Figure 1c). It is confirmed by a highly significant (
p < 0.01) linear correlation found between the FRAP values and TPC levels (
r = 0.9671), TEAC values (
r = 0.9064), and EC
50 values of the DPPH test (
r = −0.9638). The highest FRAP values were observed for EAFs (11.42–13.74 mmol Fe
2+/g, including outliers), BFs (9.81–11.47 mmol Fe
2+/g, without the outlier for
S. pogonopetala leaf), and DEFs (7.58–11.60 mmol Fe
2+/g, without the outlier for
S. wilfordii leaf). The FRAP activity of the most active extracts was comparable to the activity of RT and Trolox
®, but it is 1.5–4 times lower than those of the other analysed standards (
Table 3). For some small molecular phenolics, such as CFA, GA, and CA, their extremely high FRAP activity expressed in weight units was affected by low molecular mass. If expressed in molar units, FRAP values of these standards did not differ significantly (
p < 0.01) from the activity of RT. The observed differences in FRAP activity between small molecular phenolics and
Sorbus extracts abundant in macromolecular tannin-type proanthocyanidins (see
Section 2.3) could also be explained by different reaction kinetics of reagents differing in molecular weight [
16]. However, the slow reaction rate of plant extracts implies an ability to retain and even increase their reducing ability with time [
13,
16], and might thus signify a longer protecting effect against oxidative damage
in vivo.
The determined SET-type antioxidant activity of standards (
Table 3) was in accordance with the previous reports [
11,
16,
17] in terms of overall order and magnitude, which validated the results obtained. Some slight discrepancies may be due to the differences in the reaction conditions, such as the initial reagent concentration and analysis run time, and also because of normalisation of the EC
50 values, which was employed in the present study for DPPH and TEAC tests.
Relationships between SET-type antioxidant activity parameters of standards, although statistically significant (
p < 0.05) and linear, were weaker than those of
Sorbus extracts, which was evidenced by lower correlation coefficients for e.g., the DPPH and TEAC tests (
r = −0.8109) or the TEAC and FRAP assays (
r = 0.825). Higher
r-values found for the
Sorbus extracts indicated synergistic and additive effects of their antioxidant constituents. These effects have been documented for several other plant extracts containing phenolics, and can be explained by complementary reactivity and regeneration mechanisms between individual antioxidants, depending on their structures and on the possible formation of stable intermolecular complexes [
20].
Direct comparison of our antioxidant results with the literature data is very difficult, given the varying assay protocols utilised by different authors. On the other hand, the TPC levels are easy to compare and can be considered as an indirect measure of antioxidant activity because of the basic redox mechanism and standardised conditions of the FC method. Among the natural products, the extracts of tea leaf and grape seed appear to have the greatest antioxidant potential. The highest TPC values have been reported for the commercial ethanol extract (EE) of grape seed (60% GAE [
19]), EAFs of green tea (58% GAE [
20]) and green mate (42–48% GAE [
20]), and followed by those of 80–100% MEs (23–37% GAE [
20,
21]) obtained from the last two plant materials. There are only a few other plant extracts exhibiting comparable TPC levels, e.g., EE of
Magnifera indica leaf (59–65% GAE [
19]), ME of the
Hypericum foliosum stem (39% GAE [
22]) or
Syzygium aqueum leaf (52% GAE [
19]). In this context, the tested
Sorbus extracts appear to be very rich sources of natural antioxidants (39–58% GAE in EAFs and BFs).
2.3. Phenolic Profile of the Analysed Sorbus Dry Extracts and Fractions
It is evident that the TPC value determined by the FC assay does not give a full picture of the real phenolic constituents in plant extracts. Thus, for verification of the phenolic levels in
Sorbus, further determinations of the main phenolic groups were performed. Results of the appropriate HPLC-PDA and UV-spectrophotometric assays are reported in
Table 4 and
Table 5.
Table 4.
Total content of proanthocyanidins, hydroxybenzoic acids and flavonoids in the analysed Sorbus dry extracts and fractions a.
Table 4.
Total content of proanthocyanidins, hydroxybenzoic acids and flavonoids in the analysed Sorbus dry extracts and fractions a.
| Sample No. /Extract/ Fraction | Total proanthocyanidin content (%)
b | Hydroxybenzoic acids (%)
c | Flavonoids (%)
d |
|---|
| 1. | ME | 11.16 ± 0.37
I,J | 0.14
G,H (PCA: 0.05) | 5.83
H (SQ: 0.70; HY: 0.95; IQ: 1.67; GS: 0.94) |
| | DEF | 1.21 ± 0.05
A,B,C,D | 2.69
R (PCA: 1.65; pHBA: 0.47) | 7.92
K (HY: 1.39; IQ: 3.11; GS: 1.46; QU: 0.38) |
| | EAF | 9.81 ± 0.16
G,H | 0.10 (PCA: 0.10)
D,E,F | 36.22
T (HY: 9.02; IQ: 16.15; GS: 7.25) |
| | BF | 36.08 ± 0.59
R | 0.13
F,G | 12.26
N (SQ: 3.55; RT: 2.22; IQ: 1.12) |
| | WR | 1.95 ± 0.07
C,D,E | 0.08
B,C,D | not detected |
| 2. | ME | 8.00 ± 0.36F | 0.17
I (PCA: 0.05) | 1.67
C (HY: 0.92; IQ: 0.22; GS: 0.18) |
| | DEF | 0.57 ± 0.03
A,B | 2.19
P (PCA: 1.41; pHBA: 0.74) | 9.68
L (HY: 1.65; IQ: 0.54; GS: 0.49; QU: 1.04) |
| | EAF | 8.01 ± 0.19
F | 0.21
J (PCA: 0.15) | 21.21
S (HY: 11.60; IQ: 2.95; GS: 2.41) |
| | BF | 26.51 ± 0.61
Q | 0.22
J,K | 1.44
B,C (RT: 0.22; HY: 0.81; IQ: 0.16; GS: 0.15) |
| | WR | 0.74 ± 0.04
A,B,C | 0.14
G,H | not detected |
| 3. | ME | 10.22 ± 0.16
H,I | 0.07
B,C (PCA: 0.02) | 3.47
D,E(RT: 0.61; HY: 0.87; IQ: 0.38; GS: 0.24) |
| | DEF | 0.96 ± 0.08
A,B,C,D | 2.20
P(PCA: 1.29; pHBA: 0.40) | 5.87
H (HY: 1.66; IQ: 0.76; GS: 0.53; QU: 1.02) |
| | EAF | 10.83 ± 0.09
H,I | 0.06
A,B(PCA: 0.06) | 20.15
R (HY: 10.67; IQ: 3.91; GS: 3.20) |
| | BF | 38.36 ± 1.13
S | 0.09
C,D,E | 13.90
O (RT: 3.67; HY: 1.24; IQ: 0.78) |
| | WR | 1.10 ± 0.02
A,B,C,D | 0.06
A,B | 0.08
A |
| 4. | ME | 17.64 ± 0.42
N,O | 0.11
E,F,G (PCA: 0.06) | 1.51
B,C (RT: 0.23; HY: 0.10; GS: 0.21) |
| | DEF | 1.00 ± 0.04
A,B,C,D | 3.32
S (PCA: 2.50; pHBA: 0.77) | 1.92
C (HY: 0.31; IQ: 0.22; GS: 0.83; QU: 0.20) |
| | EAF | 13.90 ± 0.25
L | 0.22
J,K (PCA: 0.22) | 8.12
K (RT: 0.55; HY: 1.29; IQ: 0.57; GS: 3.37) |
| | BF | 46.11 ± 0.68
T | 0.21
J | 4.96
F,G (RT: 1.45; HY: 0.14) |
| | WR | 2.13 ± 0.01
D,E | 0.11
E,F | not detected |
| 5. | ME | 14.22 ± 0.32
L | 0.06
A,B (PCA: 0.03) | 3.85
E (SQ: 0.08; HY: 0.19) |
| | DEF | 0.93 ± 0.07
A,B,C,D | 0.56
L (PCA: 0.51; pHBA: 0.05) | 4.61
F (QU: 0.19) |
| | EAF | 17.13± 0.27
N | 0.05
A,B (PCA: 0.05) | 14.73
P (RT: 0.53) |
| | BF | 39.04 ± 1.10
S | not detected | 6.39
J (SQ: 0.28; HY: 0.45) |
| | WR | 0.33 ± 0.03
A | not detected | 0.53
A |
| 6. | ME | 16.81 ± 0.19
N | 0.24
K (PCA: 0.06; pHBA: 0.03) | 1.52
B,C (SQ: 0.50; RT: 0.47; HY: 0.18; GS: 0.20) |
| | DEF | 1.26 ± 0.07
A,B,C,D | 1.95
O (PCA: 0.92; pHBA: 1.03) | 1.11
B (HY: 0.34; IQ: 0.46; QU: 0.16) |
| | EAF | 15.53 ± 0.19
M | 0.23
J,K (PCA: 0.13) | 7.81
K (RT: 1.05; HY: 2.29; GS: 2.63) |
| | BF | 51.20 ± 1.24
U | 0.21
J | 5.42
G,H (SQ: 2.64; RT: 2.21) |
| | WR | 2.72 ± 0.08
E | 0.15
H,I | not detected |
| 7. | ME | 8.56 ± 0.29
F,G | 0.03
A | 3.13
D (SQ: 0.57; RT: 0.22; HY: 0.17; IQ: 0.30) |
| | DEF | 1.19 ± 0.11
G | 1.20
N (PCA: 0.72; pHBA: 0.49) | 8.04
K (IQ: 0.32; QU: 0.71) |
| | EAF | 12.36 ± 0.11
J,K | 0.07
B,C,D (PCA: 0.07) | 5.03
F,G (HY: 0.90; IQ: 1.69; QU: 0.16) |
| | BF | 20.55 ± 0.20
P | 0.12
E,F,G | 11.55
M (SQ: 3.00; RT: 1.15; IQ: 0.29) |
| | WR | 0.25 ± 0.03
A | 0.06
B,C | 0.17
A |
| 8. | ME | 12.55 ± 0.31
K | not detected | 5.55
H (SQ: 3.67; RT: 0.68) |
| | DEF | 1.61 ± 0.05
B,C,D,E | 0.89
M (PCA: 0.75; pHBA: 0.11) | 3.42
D,E (QU: 0.27) |
| | EAF | 18.62 ± 0.51
O | 0.08
B,C,D (PCA: 0.08) | 6.61
I (SQ: 0.67; RT: 0.53; IQ: 0.14) |
| | BF | 26.81 ± 0.27
Q | not detected | 18.15
Q (SQ: 13.37; RT: 2.50) |
| | WR | 1.43 ± 0.03
A,B,C,D | not detected | 0.36
A (SQ: 0.36) |
Table 5.
Total content of hydroxycinnamic acids in the analysed Sorbus dry extracts and fractions a.
Table 5.
Total content of hydroxycinnamic acids in the analysed Sorbus dry extracts and fractions a.
| Sample No. /Extract/ Fraction | Chlorogenic acid isomers (%)
b | Other caffeic acid derivatives (%)
c | p-Coumaric acid derivatives (%) d |
|---|
| 1. | ME | 6.56
I (CHA: 4.37; NCHA: 1.25; CCHA: 0.94) | 0.88
G,H (CFA: 0.02) | 0.43
M |
| | DEF | 0.26
A (CHA: 0.26) | 2.66
M (CFA: 0.51) | not detected |
| | EAF | 4.20
E,F (CHA: 3.15; NCHA: 0.51; CCHA: 0.54) | 5.48
R | not detected |
| | BF | 14.24
R (CHA: 10.41; NCHA: 1.98; CCHA: 1.85) | 0.56
D,E,F | 0.24
I |
| | WR | 6.00
H (CHA: 3.57; NCHA: 1.37; CCHA: 1.06) | 0.05
A | 0.03
A |
| 2. | ME | 9.73
P (CHA: 7.52; NCHA: 1.26; CCHA: 0.96) | 1.61
J | 0.36
L |
| | DEF | 0.64
A (CHA: 0.40; NCHA: 0.15) | 6.10
S (CFA: 1.14) | 1.28
R (pCA: 0.46) |
| | EAF | 7.76
K (CHA: 6.77; NCHA: 0.45; CCHA: 0.53) | 11.07
U | 2.07
W |
| | BF | 23.83
U (CHA: 19.49; NCHA: 2.32; CCHA: 2.02) | 2.84
M | 1.27
R |
| | WR | 7.85
K,L (CHA: 5.46; NCHA: 1.36; CCHA: 1.03) | 0.46
C,D,E | 0.10
C,D |
| 3. | ME | 9.98
P (CHA: 6.80; NCHA: 1.83; CCHA: 1.35) | 0.75
F,G | 0.12
D,E |
| | DEF | 0.69
A,B (CHA: 0.50; NCHA: 0.19) | 4.32
Q (CFA: 0.48) | 0.15
E,F |
| | EAF | 7.09
J (CHA: 5.95; NCHA: 0.50; CCHA: 0.63) | 7.31
T | 1.43
S |
| | BF | 16.46
T (CHA: 12.82; NCHA: 1.93; CCHA: 1.70) | 0.66
E,F,G | 0.40
L |
| | WR | 9.20
O (CHA: 5.51; NCHA: 2.20; CCHA: 1.50) | 0.14
A,B | 0.05
A,B |
| 4. | ME | 6.69
I,J (CHA: 5.45; NCHA: 0.47; CCHA: 0.76) | 0.59
D,E,F | 0.37
L |
| | DEF | 0.48
A (CHA: 0.33) | 2.65
M (CFA: 0.41) | 0.92
P (pCA: 0.50) |
| | EAF | 8.09
K,L,M (CHA: 7.06; NCHA: 0.29; CCHA: 0.74) | 3.76
O | 1.90
U |
| | BF | 15.48
S (CHA: 12.72; NCHA: 0.86; CCHA: 1.90) | 1.22
I | 0.99
Q |
| | WR | 5.39
G (CHA: 4.13; NCHA: 0.56; CCHA: 0.69) | 0.40
C,D | 0.12
D,E |
| 5. | ME | 2.32
D (CHA: 2.04; NCHA: 0.12; CCHA: 0.16) | 1.14
I | 0.21
H,I |
| | DEF | 0.27
A (CHA: 0.12) | 1.24
I (CFA: 0.15) | 0.91
P (pCA: 0.22) |
| | EAF | 1.16
B,C (CHA: 1.16) | 1.58
J | 0.89
P |
| | BF | 3.99
E (CHA: 3.66; CCHA: 0.33) | 1.12
H,I | 0.30
J,K |
| | WR | 2.28
D (CHA: 1.88; NCHA: 0.16; CCHA: 0.24) | 0.75
F,G | 0.03
A |
| 6. | ME | 8.72
N,O (CHA: 3.72; NCHA: 3.05; CCHA: 1.96) | 0.62
D,E,F | 0.19
G,H |
| | DEF | 0.65
A (CHA: 0.34; NCHA: 0.19; CCHA: 0.12) | 6.10
S (CFA: 0.27) | 0.91
P (pCA: 0.35) |
| | EAF | 5.36
G (CHA: 3.69; NCHA: 0.77; CCHA: 0.91) | 7.10
T | 0.79
O |
| | BF | 12.78
Q (CHA: 7.29; NCHA: 3.00; CCHA: 2.49) | not detected | 0.33
K |
| | WR | 8.26
L,M,N (CHA: 2.78; NCHA: 3.47; CCHA: 2.01) | not detected | not detected |
| 7. | ME | 5.05
G (CHA: 3.99; NCHA: 0.56; CCHA: 0.50) | 1.97
K | 0.09
C,D |
| | DEF | 0.45
A (CHA: 0.22) | 2.25
L (CFA: 0.45) | 0.78
O (pCA: 0.57) |
| | EAF | 1.47
C (CHA: 1.25; CCHA: 0.21) | 11.58
W | 0.38
L |
| | BF | 8.47
M,N (CHA: 6.99; NCHA: 0.61; CCHA: 0.87) | 1.10
H,I | 0.29
J,K |
| | WR | 4.53
F (CHA: 3.30; NCHA: 0.63; CCHA: 0.60) | not detected | not detected |
| 8. | ME | 6.54
I (CHA: 5.86; NCHA: 0.42; CCHA: 0.26) | 0.60
D,E,F | 0.17
F,G (pCA: 0.05) |
| | DEF | 0.37
A (CHA: 0.27) | 3.37
N (CFA: 0.53) | 1.50
T (pCA: 1.31) |
| | EAF | 4.07
E,F (CHA: 3.80; CCHA: 0.26) | 4.03
P | 0.52
N |
| | BF | 10.00
P (CHA: 8.94; NCHA: 0.60; CCHA: 0.46) | 0.32
B,C | 0.28
J |
| | WR | 6.58
I (CHA: 5.67; NCHA: 0.47; CCHA: 0.44) | 0.44
C,D,E | 0.07
B,C |
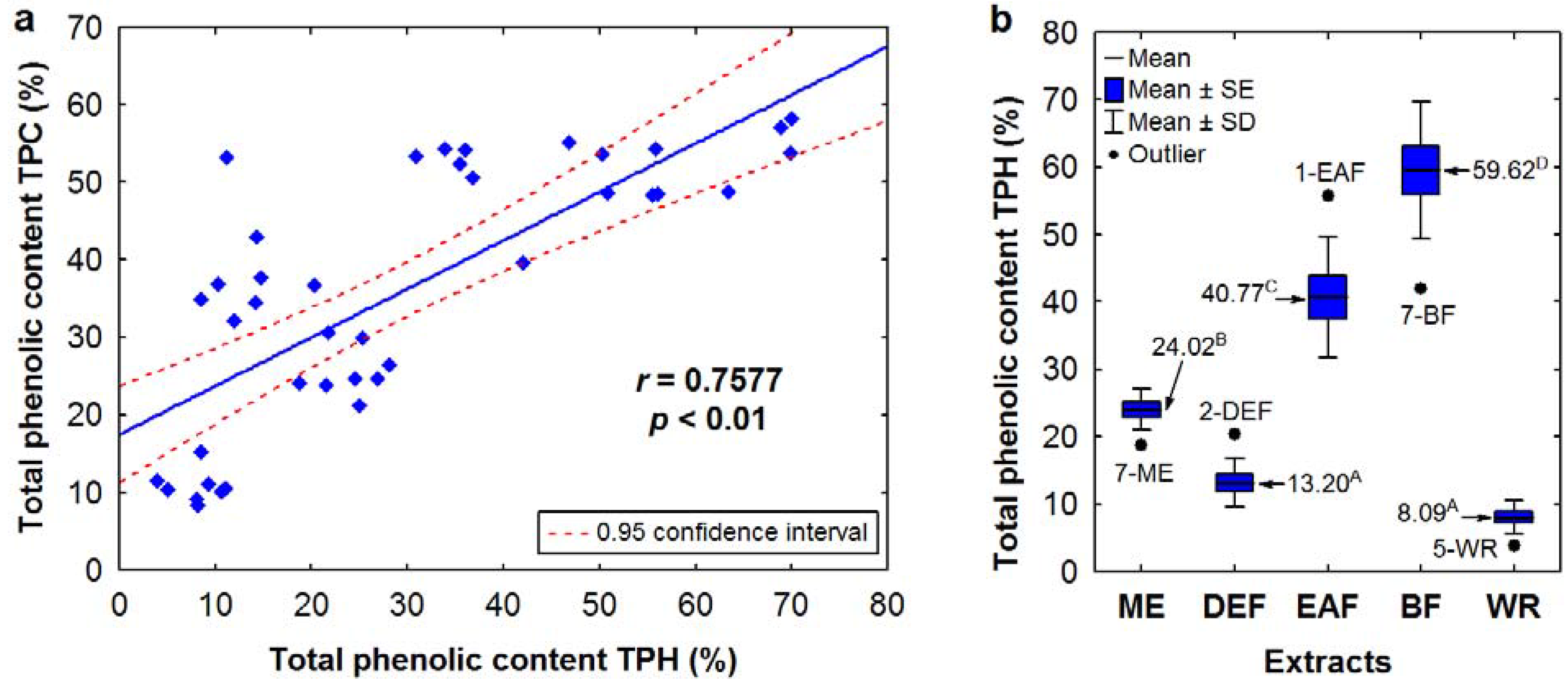
For the majority of extracts, the total phenolic content TPH, calculated as the sum of total proanthocyanidins and individual compounds quantified by HPLC, is consisted with the TPC levels expressed in GAE, which is evidenced by a high and statistically significant correlation between these parameters (
Figure 3a). Remarkable differences in these contents were observed only for DEFs, especially for leaf samples, in which the TPC values were 3–5 times higher than the TPH levels. If the DEFs were excluded, the correlation between TPC and TPH levels was stronger (
r = 0.8859,
p < 0.01).
Figure 3.
(
a) Scatter diagram of the correlation between TPC and TPH levels of the
Sorbus dry extracts. (
b) Variation in TPH levels among the
Sorbus extracts depending on the extraction solvent. Sample codes and abbreviations are given acc. to
Table 2 and
Figure 1. Values marked with different superscript letters are significantlydifferent (
p < 0.01).
Figure 3.
(
a) Scatter diagram of the correlation between TPC and TPH levels of the
Sorbus dry extracts. (
b) Variation in TPH levels among the
Sorbus extracts depending on the extraction solvent. Sample codes and abbreviations are given acc. to
Table 2 and
Figure 1. Values marked with different superscript letters are significantlydifferent (
p < 0.01).
As shown in
Figure 3b, the highest TPH levels were found for BFs (50.85–69.93% dw) with one outlier found for BF of the
S. pogonopetala leaf (42.08%) and EAFs (30.89–50.32% dw) with one outlier found for EAF of the
S. aucuparia inflorescence (55.82%). Since the same extracts were the most active SET-type antioxidants, a high and statistically significant (
p < 0.01) linear correlation was observed between the TPH contents and the EC
50 values of the DPPH test (
r = −0.7411), TEAC (
r = 0.8019) and FRAP (
r = 0.6465) values, and this is clear evidence that phenolic compounds are the most important determinants of the SET-type antioxidant activity of the tested extracts. Elimination of DEFs from the correlation test resulted in increased
r values (−0.8888, 0.8646, and 0.8008 for the DPPH, TEAC and FRAP tests), which suggested that some non-phenolic compounds could synergistically act as antioxidants in DEFs, or that the phenolics present in these extracts exhibit higher antioxidant capacity in comparison to the constituents of other extract types.
Impact of the individual phenolic groups on SET-type activity of the
Sorbus extracts was studied by multiple linear regression analysis. Apart from hydroxybenzoic acid derivatives, all other analyte groups (proanthocyanidins, flavonoids and hydroxycinnamic acid derivatives) exhibited significant (
p < 0.01) partial correlations with the activity parameters. The strongest partial correlations were found between proanthocyanidins and the TEAC values (
r = 0.6824), between flavonoids and the DPPH EC
50 (
r = 0.6086) and FRAP (
r = 0.6612) values, between
p-coumaric acid derivatives and the FRAP values (
r = 0.6058), and between total caffeic acid derivatives (including chlorogenic acid isomers) and the TEAC values (
r = 0.4789). Since the levels of
p-coumaric acid derivatives were low (0.00–2.07% dw), three other listed groups of phenolics could be deemed determinants of the tested activity. The levels of these analytes in the extracts were affected mainly by the extracting solvents as shown in the boxplots (
Figure 4a–c). The highest levels of total proanthocyanidins were found for BFs (26.51–46.00% dw, without outliers), the highest total content of caffeic acid derivatives were observed for BFs and EAFs (5.10–17.12% dw, without outliers), and the highest total flavonoid levels were found for EAFs (6.61–21.21% dw, without the outlier), which reconfirmed that ethyl acetate and
n-butanol are the best extractants of
Sorbus antioxidants.
Figure 4.
(
a–
c) Variation in the levels of main phenolic groups among the
Sorbus extracts depending on the type of extraction solvent. Sample codes and abbreviations are given acc. to
Table 2 and
Figure 1. Values marked with different superscript letters are significantly different (
p < 0.01).
Figure 4.
(
a–
c) Variation in the levels of main phenolic groups among the
Sorbus extracts depending on the type of extraction solvent. Sample codes and abbreviations are given acc. to
Table 2 and
Figure 1. Values marked with different superscript letters are significantly different (
p < 0.01).
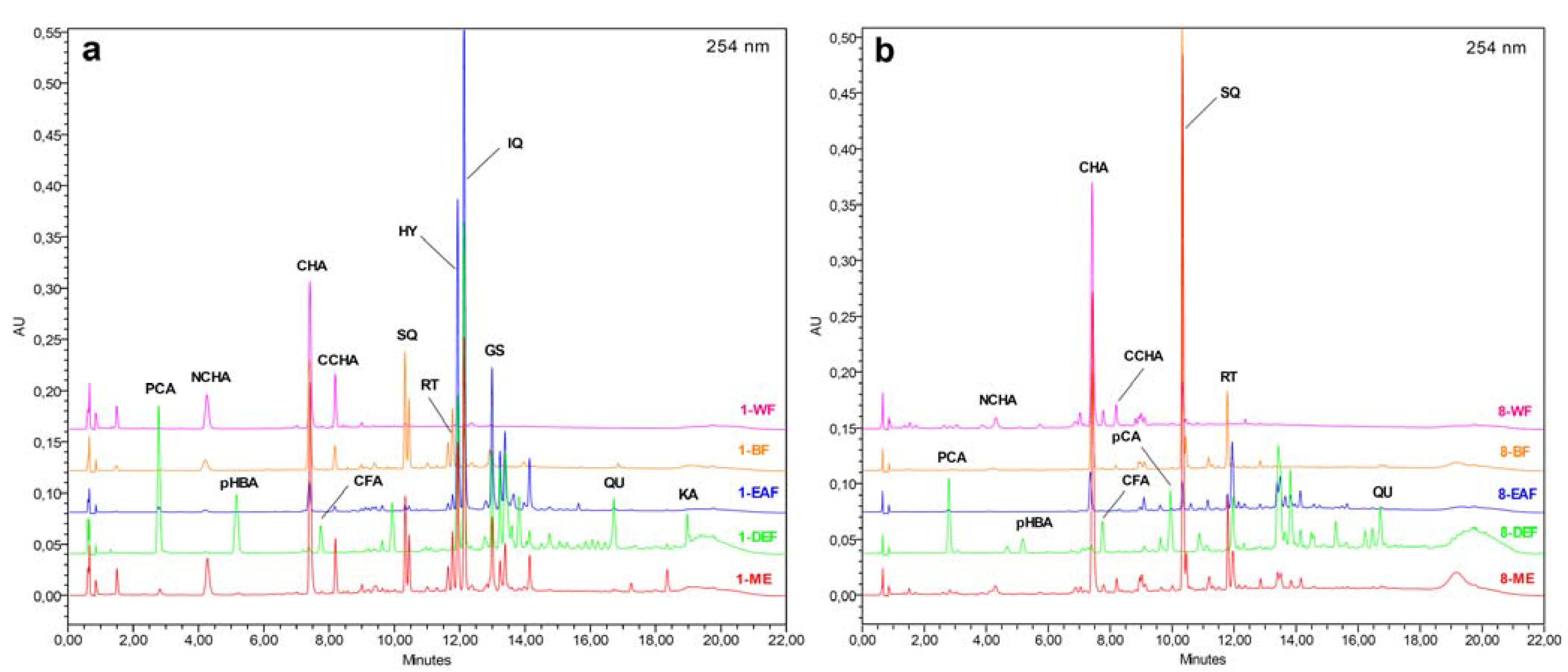
In the present work, the first time HPLC fingerprint analysis was performed for the inflorescence and leaf extracts of the tested
Sorbus species. The individual phenolic acids and flavonoids were identified by comparison of their chromatographic behaviour and PDA spectra with authentic standards, including a set of compounds isolated previously from
Sorbus plants [
23]. Apart from the fully characterised ones, several peaks were tentatively identified and classified into the appropriate groups of phenolics by their PDA spectra, which enabled quantitation of ca. 95% of the UV-absorbing constituents of the extracts. The qualitative phenolic profiles of the tested
Sorbus species appeared to be similar, and the most important interspecific differences were in quantitative levels of the individual analytes, which was exemplified for the
S. aucuparia inflorescence (
Figure 5a) and
S. wilfordii leaf (
Figure 5b). For the majority of extracts, the dominant components were identified with the standards, but in the case of DEFs of the
S. commixta inflorescence and the leaves of
S. gracilis and
S. pogonopetala, the main constituents could be only tentatively characterised and further isolation and spectroscopic studies are needed for their full structural identification.
Figure 5.
.(
a–
b) Representative HPLC fingerprint chromatograms of the
Sorbus extracts. Sample and peak codes are given acc. to
Table 2,
Table 4 and
Table 5.
Figure 5.
.(
a–
b) Representative HPLC fingerprint chromatograms of the
Sorbus extracts. Sample and peak codes are given acc. to
Table 2,
Table 4 and
Table 5.
The main components of MEs were caffeoylquinic acids (2.32–9.98% dw) and flavonoids (1.30–5.71% dw). The dominant caffeoylquinic acid in all samples was CHA, except the extract of S. koehneana inflorescence that contained considerable levels of neo- and cryptochlorogenic acids (NCHA/CCHA). The flavonoid fractions of MEs were abundant in quercetin glycosides, such as quercetin 3-O-sophoroside (SQ), RT, hyperoside (HY), isoquercitrin (IQ), and sexangularetin 3-O-β-D-glucopyranoside (GS, found only in the extracts of inflorescences). Fractionation of MEs between solvents of different polarity yielded fractions of strongly different composition. Simple phenolic acids, such as CFA, p-hydroxybenzoic (pHBA), p-coumaric (pCA), and protocatechuic (PCA) acids, as well as flavonoid aglycones, such as QU and kaempferol (KA) were found only in DEFs. On the other hand, flavonoid diglycosides (SQ, RT) were present almost exclusively in BFs (traces of these compounds could be detected in some EAFs and WRs). Flavonoid monoglycosides (HY, IQ, and GS) were found in DEFs, EAFs, and BFs, with the highest levels observed for EAFs. Caffeoylquinic acids were recorded as the major components of BFs and WRs.
Some of the analysed
Sorbus extracts turned out to be abundant in the individual phenolic metabolites, e.g., BF of the
S. commixta inflorescence in CHA (19.49% dw), EAF of the
S. aucuparia inflorescence in IQ (16.15% dw) and HY (9.05% dw), EAF of the
S. decora inflorescence in HY (10.67% dw), and BF of the
S. wilfordii leaf in SQ (13.37% dw). Apart from the strong antioxidant activity [
11,
16,
17], these compounds exhibit many other kinds of biological activities, including cholagogic, hypoglycaemic, hypotensive, anti-inflammatory, vaso- and hepatoprotective effects. This activity is a scientific basis of the use of IQ, HY, and CHA as functional food and cosmetic additives [
24]. There are, however, only a few plant extracts that accumulate high levels of these phenolics, and the richest are the extracts of green coffee beans (ca. 20% of CHA [
25]),
Equisetum arvense stems (15–38% of IQ [
26]), and
Hypericum perforatum herb (4–19% of HY [
27]). The
Sorbus extracts could thus serve as efficient source materials for isolation of pure compounds.