Synthesis, Docking Studies and Biological Evaluation of Benzo[b]thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one Derivatives on 5-HT1A Serotonin Receptors
Abstract
:1. Introduction


2. Results
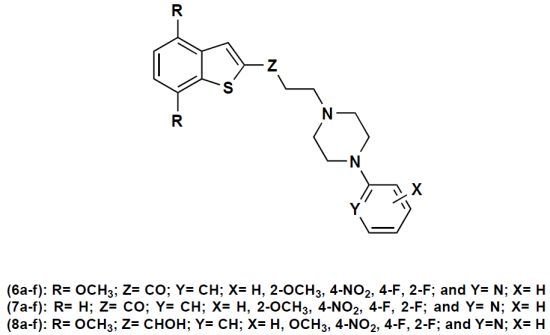
2.1. Chemistry


| Entry | Y | X | R | Yield (%) | m.p (°C) | Entry | Y | X | R | Yield (%) | m.p (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6a | CH | H | OMe | 88 | 169–170 | 7a | CH | H | H | 59 | ….. a |
| 6b | CH | 2-F | OMe | 66 | 134–135 | 7b | CH | 2-F | H | 90 | ….. b |
| 6c | CH | 4-F | OMe | 60 | 131–132 | 7c | CH | 4-F | H | 38 | 117–118 |
| 6d | CH | 2-OMe | OMe | 67 | 128–129 | 7d | CH | 2-OMe | H | 50 | 45–47 |
| 6e | N | H | OMe | 60 | 174–175 | 7e | N | H | H | 71 | 56–58 |
| 6f | CH | 4-NO2 | OMe | 65 | 161–162 | 7f | CH | 4-NO2 | H | 69 | 138–141 |

| Entry | Y | X | R | Yield (%) | m.p (°C) |
|---|---|---|---|---|---|
| 8a | CH | H | OMe | 68 | 148–149 |
| 8b | CH | 2-F | OMe | 63 | 128–129 |
| 8c | CH | 4-F | OMe | 80 | 55–56 |
| 8d | CH | 2-OMe | OMe | 93 | 55–56 |
| 8e | N | H | OMe | 77 | 157–158 |
| 8f | CH | 4-NO2 | OMe | 72 | 173–174 |
2.2. Biological Properties
| Entry | Y | X | R | Inhibition (%) | Entry | Y | X | R | Inhibition (%) |
|---|---|---|---|---|---|---|---|---|---|
| 6a | CH | H | OMe | 2 ± 2 | 7-d | CH | 2-OMe | H | 52 ± 7 |
| 6b | CH | 2-F | OMe | 14 ± 2 | 7-e | N | H | H | 60 ± 4 |
| 6c | CH | 4-F | OMe | 0 ± 3 | 7-f | CH | 4-NO2 | H | 0 ± 4 |
| 6d | CH | 2-OMe | OMe | 44 ± 2 | 8-a | CH | H | OMe | 24 ± 4 |
| 6e | N | H | OMe | 17 ± 5 | 8-b | CH | 2-F | OMe | 21 ± 1 |
| 6f | CH | 4-NO2 | OMe | 0 ± 3 | 8-c | CH | 4-F | OMe | 23 ± 3 |
| 7a | CH | H | H | …… a | 8-d | CH | 2-OMe | OMe | 38 ± 0 |
| 7b | CH | 2-F | H | 33 ± 3 | 8-e | N | H | OMe | 27 ± 3 |
| 7c | CH | 4-F | H | 31 ± 7 | 8-f | CH | 4-NO2 | OMe | 14 ± 2 |
2.3. Docking Studies
2.3.1. Molecular Modeling Studies of 5-HT1AR in complex with Ligands 6d, 6f, 7f, 7e
| Residues 5-HT1AR | Ligands | |||
|---|---|---|---|---|
| 6d | 6f | 7f | 7e | |
| D116 | + | + | + | +, ¥ |
| F360 | Y | Y | Y | Ya |
| F361 | Y | - | - | NOI |
| L380 | NOI | - | - | + |
| N385 | NOI | |||

3. Experimental
3.1. General
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of 1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanone Derivatives 6a–f
3.2.2. Synthesis of 1-(Benzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanone Derivatives 7a–f
3.2.3. General Procedure for the Preparation of 1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanol Derivatives 8a–f
3.3. Biological Methods
Radioligand Binding Assays
3.4. Molecular Modeling
3.4.1. 5.HT1A Receptor Modeling
3.4.2. Molecular Docking
3.4.3. Binding Model Analysis
4. Conclusions
Acknowledgments
References and Notes
- Caliendo, G.; Santagada, V.; Perisutti, E.; Fiorino, F. Derivatives as 5HT1A receptor ligands—Past and present. Curr. Med. Chem. 2005, 12, 1721–1735. [Google Scholar] [CrossRef]
- Elhwuegi, A.S. Progress in neuro-psychopharmacology and biological psychiatry. Prog. Neuro-Psychoph. 2004, 28, 435–451. [Google Scholar] [CrossRef]
- Czopek, A.; Byrtus, H.; Kołaczkowski, M.; Pawłowski, M.; Dybała, M.; Nowak, G.; Tatarczynska, E.; Wesołowska, A.; Chojnacka-Wójcik, E. Synthesis and pharmacological evaluation of new 5-(cyclo)alkyl-5-phenyl-and 5-spiroimidazolidine-2,4-dione derivatives. Novel 5-HT1A receptor agonist with potential antidepressant and anxiolytic activity. Eur. J. Med. Chem. 2010, 45, 1295–1303. [Google Scholar]
- Herold, F.; Chodkowski, A.; Izbicki, Ł.; Król, M.; Kleps, J.; Tur1o, J.; Nowak, G.; Stachowicz, K.; Dybała, M.; Siwek, A. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity, Part 1. Eur. J. Med. Chem. 2009, 44, 1710–1717. [Google Scholar] [CrossRef]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar]
- Akimova, E.; Lanzenberger, R.; Kasper, S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiat. 2009, 66, 627–635. [Google Scholar] [CrossRef]
- Dounay, A.B.; Barta, N.S.; Bikker, J.A.; Borosky, S.A.; Campbell, B.M.; Crawford, T.; Denny, L.; Evans, L.M.; Gray, D.L.; Lee, P.; et al. Synthesis and pharmacological evaluation of aminopyrimidine series of 5-HT1A partial agonists. Bioorg. Med. Chem. Lett. 2009, 19, 1159–1163. [Google Scholar]
- Pessoa-Mahana, H.; Araya-Maturana, R.; Saitz, B.C.; David Pessoa-Mahana, C. A synthetic overview of antidepressants with 5-HT1A binding affinities. Mini. Rev. Med. Chem. 2003, 3, 77–93. [Google Scholar] [CrossRef]
- Bromidge, S.M.; Bertani, B.; Borriello, M.; Bozzoli, A.; Faedo, S.; Gianotti, M.; Gordon, L.J.; Hill, M.; Zucchelli, V.; Watson, J.M.; et al. 8-[2-(4-Aryl-1-piperazinyl)ethyl]-2H-1,4-benzoxazin-3(4H)-ones: Dual-acting 5-HT1A/B/D receptor antagonists and serotonine re-uptake inhibitors. Part II. Bioorg. Med. Chem. Lett. 2009, 19, 2338–2342. [Google Scholar] [CrossRef]
- Butini, S.; Campiani, G.; Franceschini, S.; Trotta, F.; Kumar, V.; Guarino, E.; Borrelli, G.; Fiorini, I.; Novellino, E.; Fattorusso, C.; et al. Discovery of bishomo(hetero)arylpiperazines as novel multifunctional ligands targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors. J. Med. Chem. 2010, 53, 4803–4807. [Google Scholar] [CrossRef]
- Martínez-Esparza, J.; Oficialdegui, A.M.; Pérez-Silanes, S.; Heras, B.; Orús, L.; Palop, J.A.; Lasheras, B.; Roca, J.; Mourelle, M.; Bosch, A.; et al. New 1-aryl-3-(4-arylpiperazin-1-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. J. Med. Chem. 2001, 44, 418–428. [Google Scholar] [CrossRef]
- Orús, L.; Pérez-Silanes, S.; Oficialdegui, A.M.; Martínez-Esparza, J.; Del Castillo, J.C.; Mourelle, M.; Langer, T.; Guccione, S.; Donzella, G.; Krovat, E.M.; et al. Synthesis and molecular modeling of new 1-aryl-3-[4-(arylpiperazin-1-yl]-1-propane derivatives with high affinity at the serotonin transporter and at 5-HT1A receptors. J. Med. Chem. 2002, 45, 4128–4139. [Google Scholar]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel benzo[b]thiophene derivatives as new potential antidepressants with rapid onset of action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef]
- Pessoa-Mahana, H.; González, C.M.; González, S.M.; Pessoa-Mahana, C.D.; Araya-Maturana, R.; Ron, H.N.; Saitz, B.C. Solvent-free microwave-promoted Michael addition of aza-nucleophiles to benzo[b]thiophen-2-yl-2-propenone. ARKIVOC 2009, xi, 316–325. [Google Scholar]
- Pessoa-Mahana, H.; Kosche, C.J.; Ron, H.N.; Recabarren-Gajardo, G.; Saitz, B.C.; Araya-Maturana, R.; Pessoa-Mahana, C.D. Solvent-free microwave synthesis of 3-[4-benzo[b]thiophene-2-carbonyl)-1-piperazynil-1-benzo[b]thiophen-2-yl-1-propanones. New hetero bis-ligands with potential 5-HT1A serotonergic activity. Heterocycles 2008, 75, 1913–1929. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Zlatovic, M.V.; Sukalovic, V.V.; Schneider, C.; Roglic, G.M. Interaction of arylpiperazine ligands with the hydrophobic part of the 5-HT1A receptor binding Site. Bioorg. Med. Chem. 2006, 14, 2994–3001. [Google Scholar] [CrossRef]
- Santana, L.; Uriarte, E.; Fall, Y.; Teijeira, M.; Teran, C.; Garcia-Martinez, E.; Tolf, B.R. Synthesis and structure–activity relationships of new arylpiperazines: para substitution with electron-withdrawing groups decrease binding to 5-HT1A and D2A receptors. Eur. J. Med. Chem. 2002, 37, 503–510. [Google Scholar] [CrossRef]
- Martinez, J.; Perez, S.; Oficialdegui, A.M.; Heras, B.; Orus, L.; Villanueva, H.; Palop, J.A.; Roca, J.; Mourelle, M.; Bosch, A.; et al. New 3-[4-(aryl)piperazin-1-yl]-1-(benzo[b]thiophen-3-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. Eur. J. Med. Chem. 2001, 36, 55–61. [Google Scholar] [CrossRef]
- Glennon, R.A.; Naiman, N.A.; Pierson, M.E.; Titeler, M.; Lyon, R.A.; Weisberg, E. NAN-190: An arylpiperazine analog that antagonizes the stimulus effects of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). Eur. J. Pharmacol. Mol. Pharmacol. 1988, 154, 339–341. [Google Scholar]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Tortorella, V.; Fiorentini, F.; Olgiati, V.; Vanotti, E.; Govoni, S. Mixed 5-HT1A/D-2 activity of a new model of arylpiperazines: 1-Aryl-4-[3-(1,2-dihydronaphthalen-4-yl)-n-propyl]piperazines. 1. Synthesis and structure-activity relationship. J. Med. Chem. 1994, 37, 99–104. [Google Scholar] [CrossRef]
- Mokrosz, J.L.; Paluchowska, M.H.; Chojnacka-Wojcik, E.; Filip, M.; Charakchieva-Minol, S.; Deren-Wesolek, A.; Mokrosz, M.J. Structure-activity relationship studies of central nervous system agents. 13. 4-[3-(Benzotriazol1-1-y1)propyll-1-(2 methoxyphenyl)piperazine,a new putative 5-HT1A receptor antagonist, and its analogs. J. Med. Chem. 1994, 37, 2754–2760. [Google Scholar] [CrossRef]
- Oficialdegui, A.M.; Martinez, J.; Perez, S.; Heras, B.; Irurzun, M.; Palop, J.A.; Tordera, R.; Lasheras, B.; del Rio, J.; Monge, A. Design, synthesis and biological evaluation of new 3-[(4-aryl)piperazin-1-yl]-1-arylpropane derivatives as potential antidepressants with a dual mode of action: Serotonin reuptake inhibition and 5-HT1A receptor antagonism. Farmaco 2000, 55, 345–353. [Google Scholar] [CrossRef]
- Orús, L.; Sainz, Y.; Perez, S.; Oficialdegui, A.M.; Martinez, J.; Lasheras, B.; del Río, J.; Monge, A. New 3-[4-(aryl)piperazin-1-yl]-1-(benzo[b]thiophen-2-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. Pharmazie 2002, 57, 355–357. [Google Scholar]
- Leopoldo, M.; Lacivita, E.; Colabufo, N.A.; Niso, M.; Berardi, F.; Perrone, R. Bivalent ligand approach on 4-[2-(3-Methoxyphenyl)ethyl]-1-(2-methoxyphenyl) piperazine: Synthesis and binding affinities for 5-HT7 and 5-HT1A receptors. Med. Chem. Lett. 2007, 15, 5316–5321. [Google Scholar]
- Srinivas, B.N.; Subhash, M.N.; Vinod, K.Y. Cortical 5-HT(1A) receptor downregulation by antidepressants in rat brain. Neurochem. Int. 2001, 38, 573–579. [Google Scholar] [CrossRef]
- Bairoch, A.A.R.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; Martin, M.J.; et al. The Universal Protein Resource (UniProt). Nucleic Acids Res. 2005, 33, 154–159. [Google Scholar]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef]
- Shpaer, E.G.; Robinson, M.; Yee, D.; Candlin, J.D.; Mines, R.; Hunkapiller, T. Sensitivity and selectivity in protein similarity searches. Genomics 1996, 38, 179–191. [Google Scholar]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, D.J.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 1983, 4, 187–217. [Google Scholar]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Morris, A.L.; MacArthur, M.W.; Hutchinson, E.G.; Thornton, J.M. Stereochemical quality of protein structure coordinates. Proteins 1992, 12, 345–364. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a lamarckian genetic algorithm and and empirical binding free energy function. J. Comp. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 6-b, 8-a, 8-d, 8-e are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pessoa-Mahana, H.; Recabarren-Gajardo, G.; Temer, J.F.; Zapata-Torres, G.; Pessoa-Mahana, C.D.; Barría, C.S.; Araya-Maturana, R. Synthesis, Docking Studies and Biological Evaluation of Benzo[b]thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one Derivatives on 5-HT1A Serotonin Receptors. Molecules 2012, 17, 1388-1407. https://doi.org/10.3390/molecules17021388
Pessoa-Mahana H, Recabarren-Gajardo G, Temer JF, Zapata-Torres G, Pessoa-Mahana CD, Barría CS, Araya-Maturana R. Synthesis, Docking Studies and Biological Evaluation of Benzo[b]thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one Derivatives on 5-HT1A Serotonin Receptors. Molecules. 2012; 17(2):1388-1407. https://doi.org/10.3390/molecules17021388
Chicago/Turabian StylePessoa-Mahana, Hernán, Gonzalo Recabarren-Gajardo, Jenny Fiedler Temer, Gerald Zapata-Torres, C. David Pessoa-Mahana, Claudio Saitz Barría, and Ramiro Araya-Maturana. 2012. "Synthesis, Docking Studies and Biological Evaluation of Benzo[b]thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one Derivatives on 5-HT1A Serotonin Receptors" Molecules 17, no. 2: 1388-1407. https://doi.org/10.3390/molecules17021388