1,3-Bisdiphenylethenyl-substituted Carbazolyl Derivatives as Charge Transporting Materials
Abstract
:1. Introduction
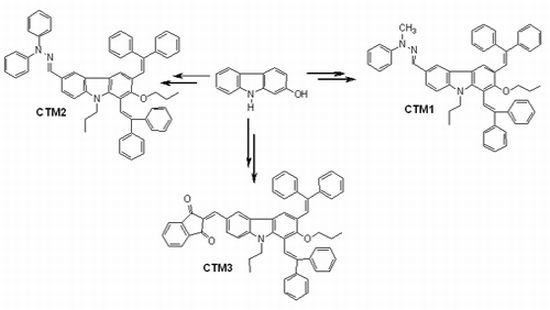
2. Results and Discussion
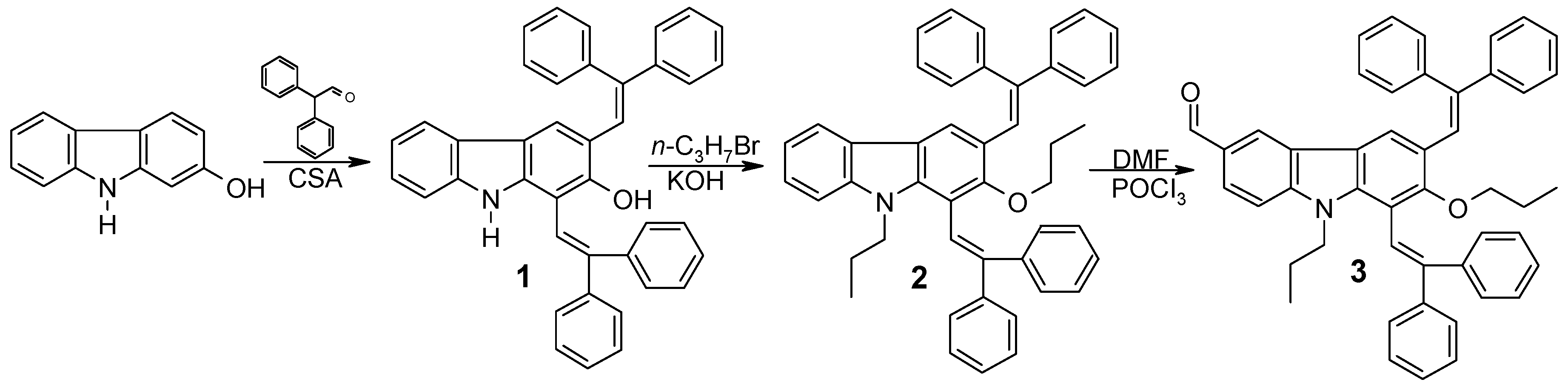
2.1. Synthesis

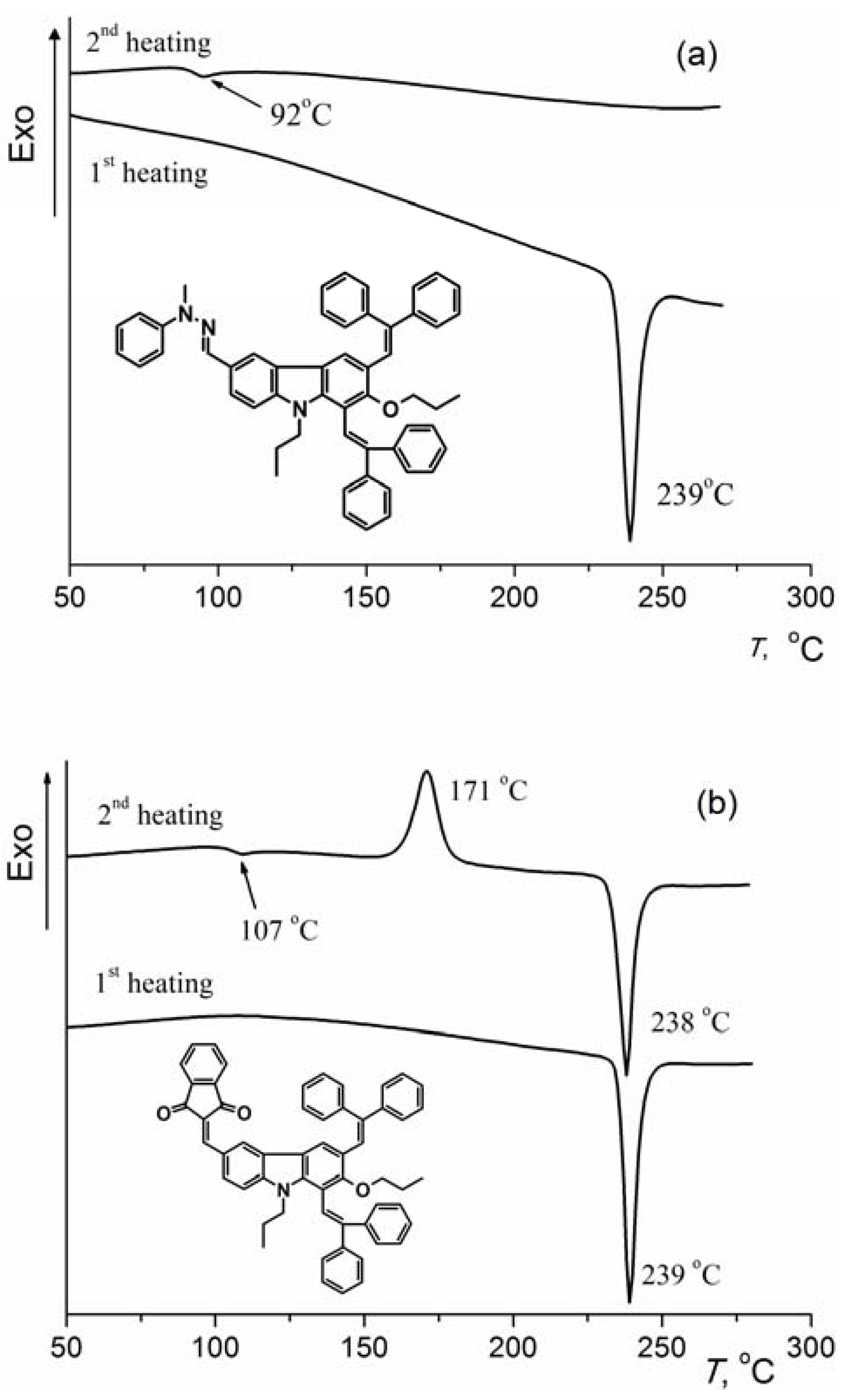
2.2. Thermal Properties
| Compound | 2 | CTM1 | CTM2 | CTM3 |
|---|---|---|---|---|
| Tm (°C) | 144 | 239 | 220 | 239 |
| Tg (°C) | 64 | 92 | 94 | 107 |
2.3. UV-Vis Spectra
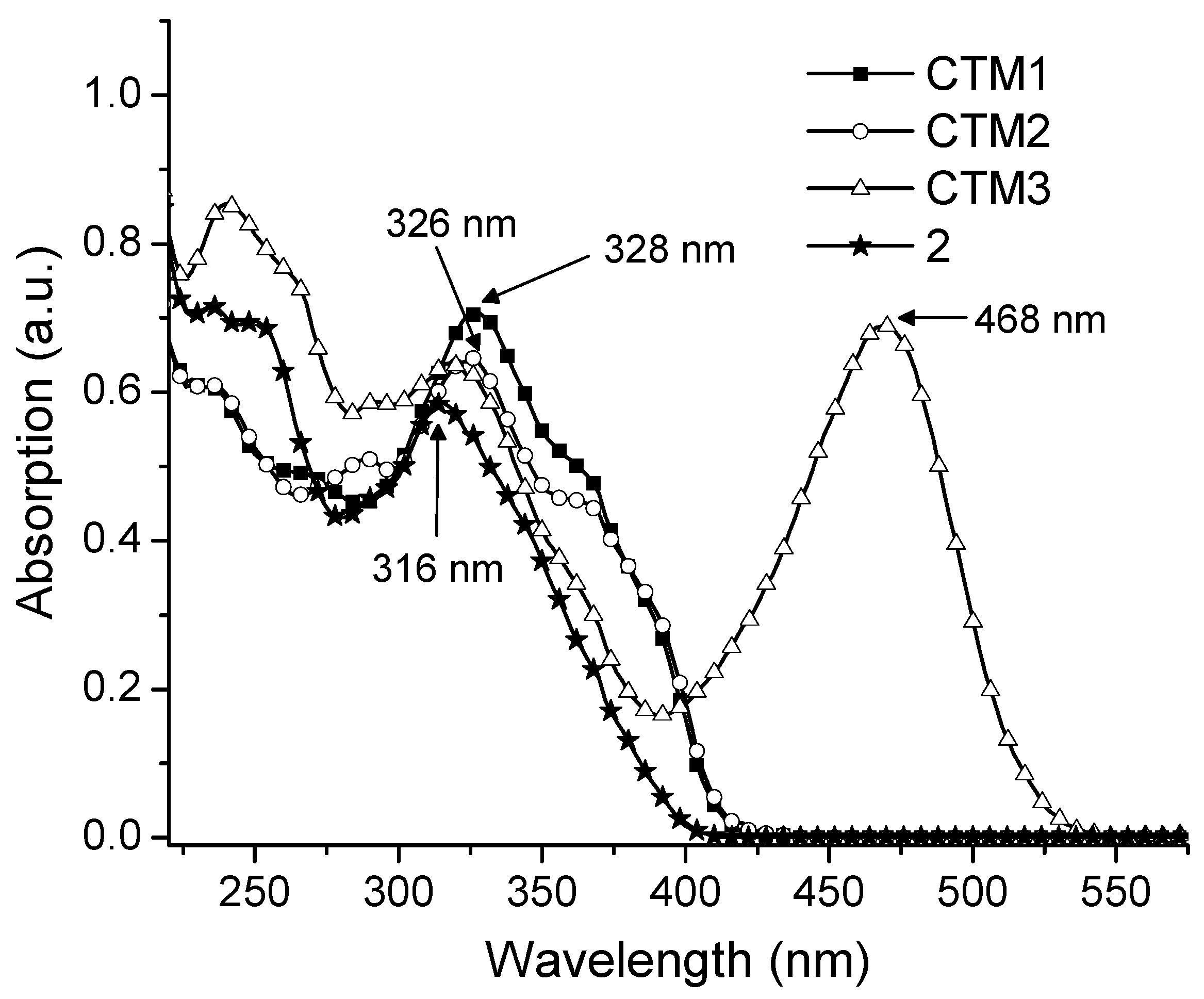
2.4. Photophysical Properties

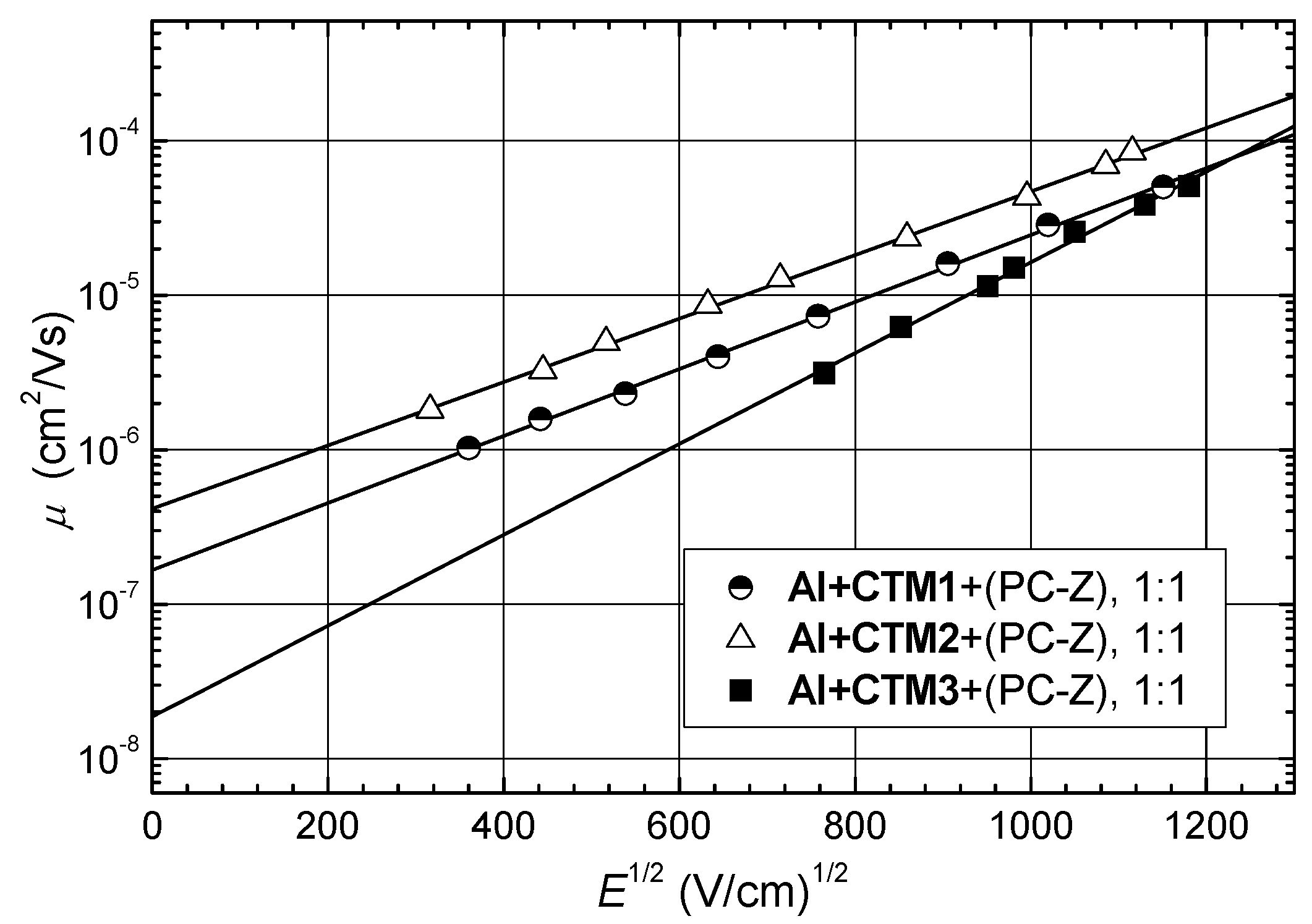
| Compound | Ip (eV) | Layer composition | Layer thickness (μm) | μo (cm2/V·s) | μ (cm2/V·s) 1 |
|---|---|---|---|---|---|
| CTM1 | 5.90 | Al+(CTM1+PC-Z, 1:1) | 9 | 4·10−7 | 1.8·10−5 |
| CTM2 | 5.87 | Al+(CTM2+PC-Z, 1:1) | 8 | 1.6·10−7 | 9·10−6 |
| CTM3 | 5.54 | Al+(CTM3+PC-Z, 1:1) | 5.4 | 2·10−8 | 4·10−6 |
3. Experimental
3.1. General
3.2. Synthesis Procedures
3.3. Measurements
3.3.1. Ionization Potential Measurement
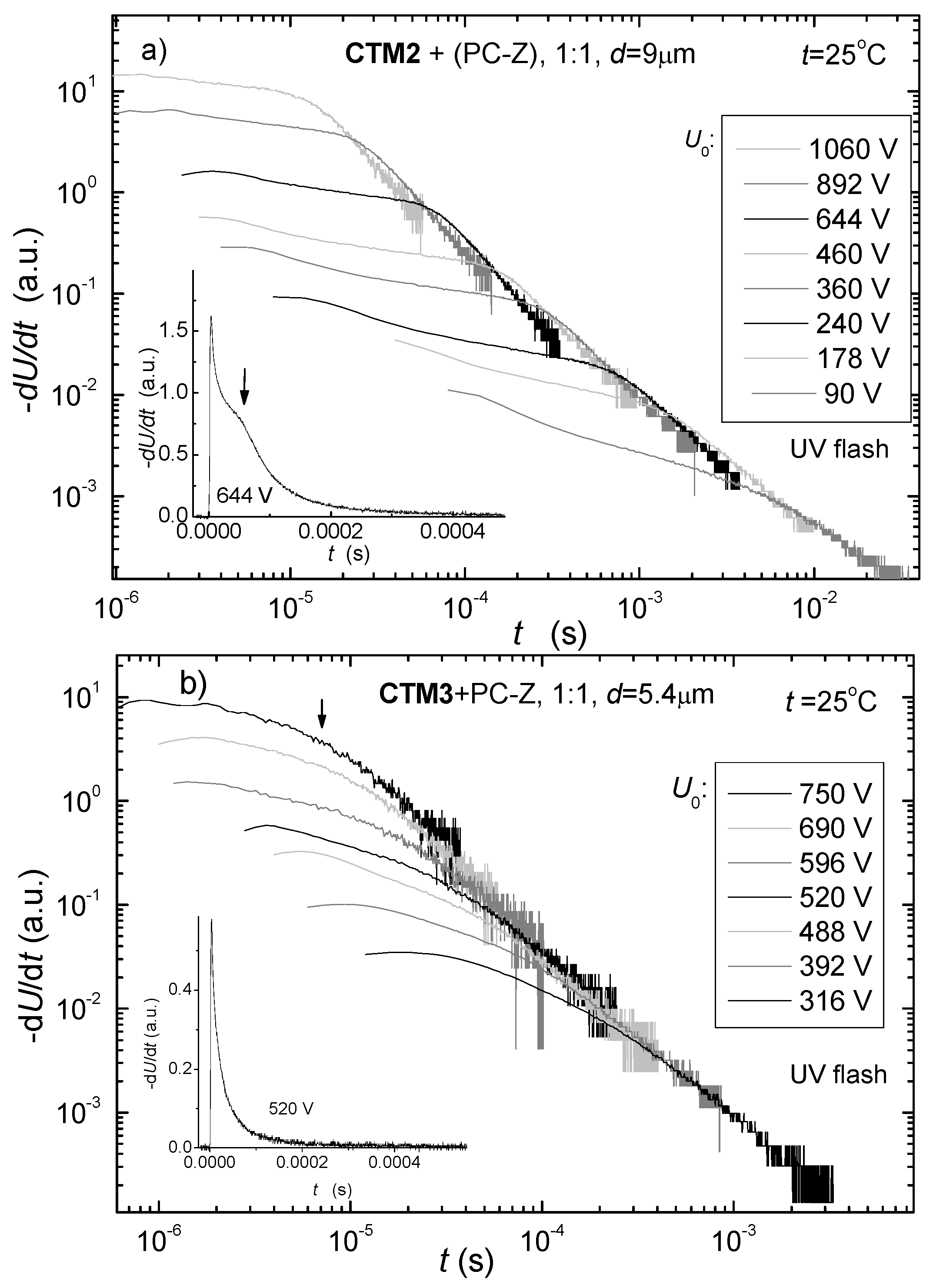
3.3.2. Charge Transport Measurement
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References
- Hoegl, H. On Photoelectric Effects in Polymers and Their Sensitization by Dopants. J. Phys. Chem. 1965, 69, 755–766. [Google Scholar] [CrossRef]
- Grazulevicius, V.J.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing Polymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; MacKay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. [Google Scholar]
- Ostroverkhova, O.; Moerner, W.E. Organic Photorefractives: Mechanisms, Materials, and Applications. Chem. Rev. 2004, 104, 3267–3314. [Google Scholar] [CrossRef]
- Weiss, D.S.; Abkowitz, M. Advances in Organic Photoconductor Technology. Chem. Rev. 2010, 110, 479–526. [Google Scholar] [CrossRef]
- Akcelrud, L. Electroluminescent Polymers. Prog. Polym. Sci. 2003, 28, 875–962. [Google Scholar]
- Li, Z.; Wang, L.; Xiong, B.; Ye, C.; Qin, J.; Li, Z. Novel, side-on, PVK-based Nonlinear Optical Polymers: Synthesis and NLO. Dyes Pigm. 2010, 84, 134–139. [Google Scholar] [CrossRef]
- Wakim, S.; Beaupré, S.; Blouin, N.; Aich, B.R.; Rodman, S.; Gaudiana, R.; Tao, Y.; Leclerc, M. Highly Efficient Organic Solar Cells Based on a Poly(2,7-carbazole) Derivative. J. Mater. Chem. 2009, 19, 5351–5358. [Google Scholar] [CrossRef]
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for Flexible Displays: From Material Selection to Device Applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Bubniene, G.; Malinauskas, T.; Daskeviciene, D.; Jankauskas, V.; Getautis, V. Easily Functionalizable Carbazole Based Building Blocks with Extended Conjugated Systems for Optoelectronic Applications. Tetrahedron 2010, 66, 3199–3206. [Google Scholar] [CrossRef]
- Stumbraite, J.; Daskeviciene, M.; Degutyte, R.; Jankauskas, V.; Getautis, V. Synthesis of Aryl(hetero)methylene-1,3-indandione Based Molecular Glasses. Monatshefte für Chemie 2007, 138, 1243–1248. [Google Scholar] [CrossRef]
- Lygaitis, R.; Getautis, V.; Grazulevicius, V.J. Hole-Transporting Hydrazones. Chem. Soc. Rev. 2008, 37, 770–788. [Google Scholar] [CrossRef]
- Janeliunas, D.; Daskeviciene, M.; Getautis, V.; Gaidelis, V.; Jankauskas, V.; Sidaravicius, J. Electron Transporting Molecular Glasses Based on Arylmethylene-1,3-Indandione. Mol. Cryst. Liq. Cryst. 2008, 497, 173–185. [Google Scholar]
- Miyamoto, E.; Yamaguchi, Y.; Yokoyama, M. Ionization potential of organic pigment film by atmospheric photoelectron emission analysis. Electrophotography 1989, 28, 364–370. [Google Scholar]
- Daskeviciene, M.; Getautis, V.; Grazulevicius, V.J.; Stanisauskaite, A.; Antulis, J.; Gaidelis, V.; Jankauskas, V.; Sidaravicius, J. Crosslinkable Carbazolyl-Containing Molecular Glasses for Electrophotography. J. Imaging Sci. Technol. 2002, 46, 467–472. [Google Scholar]
- Montrimas, E.; Gaidelis, V.; Pazera, A. The Discharge Kinetics of Negatively Charged Se Electrophotographic Layers. Lithuanian J. Phys. 1966, 6, 569–578. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bubniene, G.; Daskeviciene, M.; Pukalskas, A.; Jankauskas, V.; Getautis, V. 1,3-Bisdiphenylethenyl-substituted Carbazolyl Derivatives as Charge Transporting Materials. Molecules 2012, 17, 14846-14857. https://doi.org/10.3390/molecules171214846
Bubniene G, Daskeviciene M, Pukalskas A, Jankauskas V, Getautis V. 1,3-Bisdiphenylethenyl-substituted Carbazolyl Derivatives as Charge Transporting Materials. Molecules. 2012; 17(12):14846-14857. https://doi.org/10.3390/molecules171214846
Chicago/Turabian StyleBubniene, Giedre, Maryte Daskeviciene, Audrius Pukalskas, Vygintas Jankauskas, and Vytautas Getautis. 2012. "1,3-Bisdiphenylethenyl-substituted Carbazolyl Derivatives as Charge Transporting Materials" Molecules 17, no. 12: 14846-14857. https://doi.org/10.3390/molecules171214846