Efficacy of Carbazole Alkaloids, Essential Oil and Extract of Murraya koenigii in Enhancing Subcutaneous Wound Healing in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.1.1. Identification and Structure Elucidation of Carbazole Alkaloids

2.1.2. Profiling of the Essential Oil
| RT (min) | Ref RI | RI | Volatile Compound | Concentration (%) |
|---|---|---|---|---|
| 15.73 | 1082 a | 1079 | Linalol | 0.56 |
| 15.91 | 1099 b | 1095 | trans-Sabinene hydrate | 0.53 |
| 17.01 | 1109 a | 1112 | trans-2-Cyclohexen-1-ol | 0.48 |
| 17.88 | 1110 a | 1113 | cis-2-Cyclohexen-1-ol | 0.54 |
| 19.70 | 1189 b | 1185 | para-Cymen-8-ol | 10.31 |
| 20.42 | 1143 b | 1139 | β-Terpineol | 2.52 |
| 21.03 | 1175 a | 1170 | trans-Piperitol | 0.40 |
| 21.74 | 1276 a | 1273 | Chrysanthenyl acetate | 0.39 |
| 24.16 | 1284 b | 1279 | Lavandulyl acetate | 1.67 |
| 24.37 | 1285 b | 1285 | Bornyl acetate | 1.68 |
| 28.31 | 1375 b | 1370 | α-Copaene | 0.82 |
| 28.91 | 1390 b | 1385 | β-Elemene | 0.35 |
| 29.39 | 1394 a | 1390 | (Z)-Jasmone | 0.11 |
| 30.29 | 1494 a | 1489 | β-Caryophyllene | 19.50 |
| 31.09 | 1438 b | 1436 | Aromadendrene | 0.72 |
| 31.84 | 1454 b | 1448 | α-Humulene | 15.24 |
| 32.70 | 1420 a | 1425 | Butanedioic acid | 2.18 |
| 33.29 | 1487 b | 1480 | β-Selinene | 3.81 |
| 33.30 | 1470 a | 1472 | Naphthalene | 1.90 |
| 33.55 | 1474 a | 1478 | α-Selinene | 6.10 |
| 34.37 | 1518 b | 1512 | δ-Cadinene | 2.03 |
| 36.03 | 1562 b | 1566 | Nerolidol | 2.64 |
| 36.05 | 1564 b | 1569 | trans-Nerolidol | 1.32 |
| 36.28 | 1475 a | 1481 | Cycloheptane | 0.13 |
| 36.92 | 1576 b | 1580 | Spathulenol | 1.98 |
| 37.13 | 1587 b | 1591 | Caryophyllene oxide | 2.14 |
| 37.26 | 1594 b | 1590 | Viridiflorol | 1.51 |
| 38.13 | 1598 a | 1592 | 2-Naphthalenemethanol | 0.66 |
| 38.26 | 1079 b | 1074 | Trivertal | 0.35 |
| 38.55 | 1696 b | 1694 | Juniper camphor | 1.57 |
| 38.83 | 1581 b | 1579 | Cubenol | 0.57 |
| 39.44 | 1472 a | 1476 | β-Cadina-1(6),4-diene | 0.50 |
| 40.16 | 1593 a | 1596 | Selina-6-en-4-ol | 4.78 |
| 54.95 | 2106 b | 2105 | Phytol | 10.07 |
| Composition of grouped volatile compounds (%) | ||||
| Monoterpenes (oxygenated) | 35.29 | |||
| Sesquiterpenes (hydrocarbon) | 35.29 | |||
| Sesquiterpenes (oxygenated) | 26.47 | |||
| Diterpenes (oxygenated) | 2.94 | |||
2.2. Biology
2.2.1. Wound Contraction
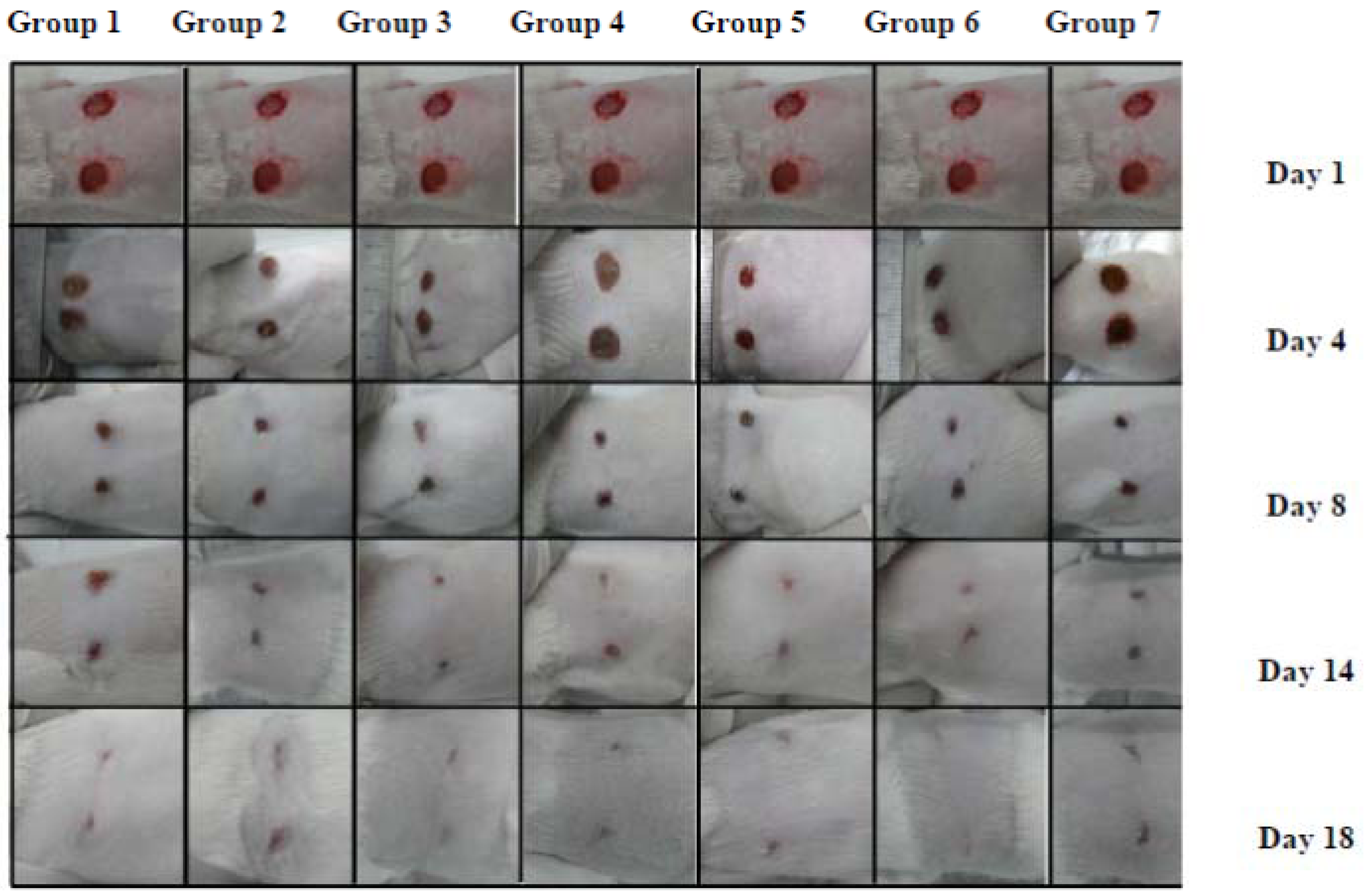
| Group | Post-wounding days | Epithelialization period (days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 17 | 18 | ||
| 1 | 2.12 ± 4.12 | 6.25 ± 4.12 | 10.6 ± 2.93 | 15.9 ± 3.66 | 25.8 ± 4.09 | 40 ± 2.26 | 57.5 ± 1.78 | 82.7 ± 2.13 | 90 ± 1.22 | 100 | 18 |
| 2 | 4.6 ± 3.77 | 8.6 ± 4.04 | 10.7 ± 3.16 | 18.0 ± 3.21 | 35 ± 2.34 | 45.5 ± 2.11 | 62 ± 1.08 | 88.5 ± 2.03 | 96 ± 1.35 | 100 | 18 |
| 3 | 6.25 ± 2.42 | 12.6 ± 3.92 | 22.0 ± 3.12 | 27.0 ± 3.24 | 38 ± 4.05 | 52.5 ± 1.09 | 68.5 ± 1.87 | 90.7 ± 2.88 | 98 ± 1.30 | 100 | 18 |
| 4 | 6.5 ± 3.72 | 6.5 ± 3.88 | 12.5 ± 4.86 | 20 ± 3.56 | 39.5 ± 3.75 | 50.5 ± 0.98 | 65 ± 1.88 | 93 ± 2.04 | 97.2 ± 1.05 | 100 | 18 |
| 5 | 5.5 ± 3.03 | 7.5 ± 4.13 | 10.7 ± 5.67 | 17.5 ± 2.61 | 36 ± 4.23 | 46 ± 2.57 | 63 ± 1.09 | 92.5 ± 2.45 | 95.5 ± 0.84 | 100 | 18 |
| 6 | 6.25 ± 2.42 | 19.25 ± 3.12 | 37.9 ± 3.09 | 49.5 ± 1.09 | 67.5 ± 1.01 | 75 ± 0.78 | 95.5 ± 0.66 | 100 ± 0.55 | - | - | 16 |
| 7 | 3.5 ± 3.11 | 7.7 ± 2.02 | 17.9 ± 2.08 | 20.5 ± 3.34 | 37.8 ± 3.12 | 45 ± 2.11 | 61 ± 0.76 | 90 ± 0.84 | 95 ± 0.83 | 100 | 18 |
2.2.2. Collagen Density
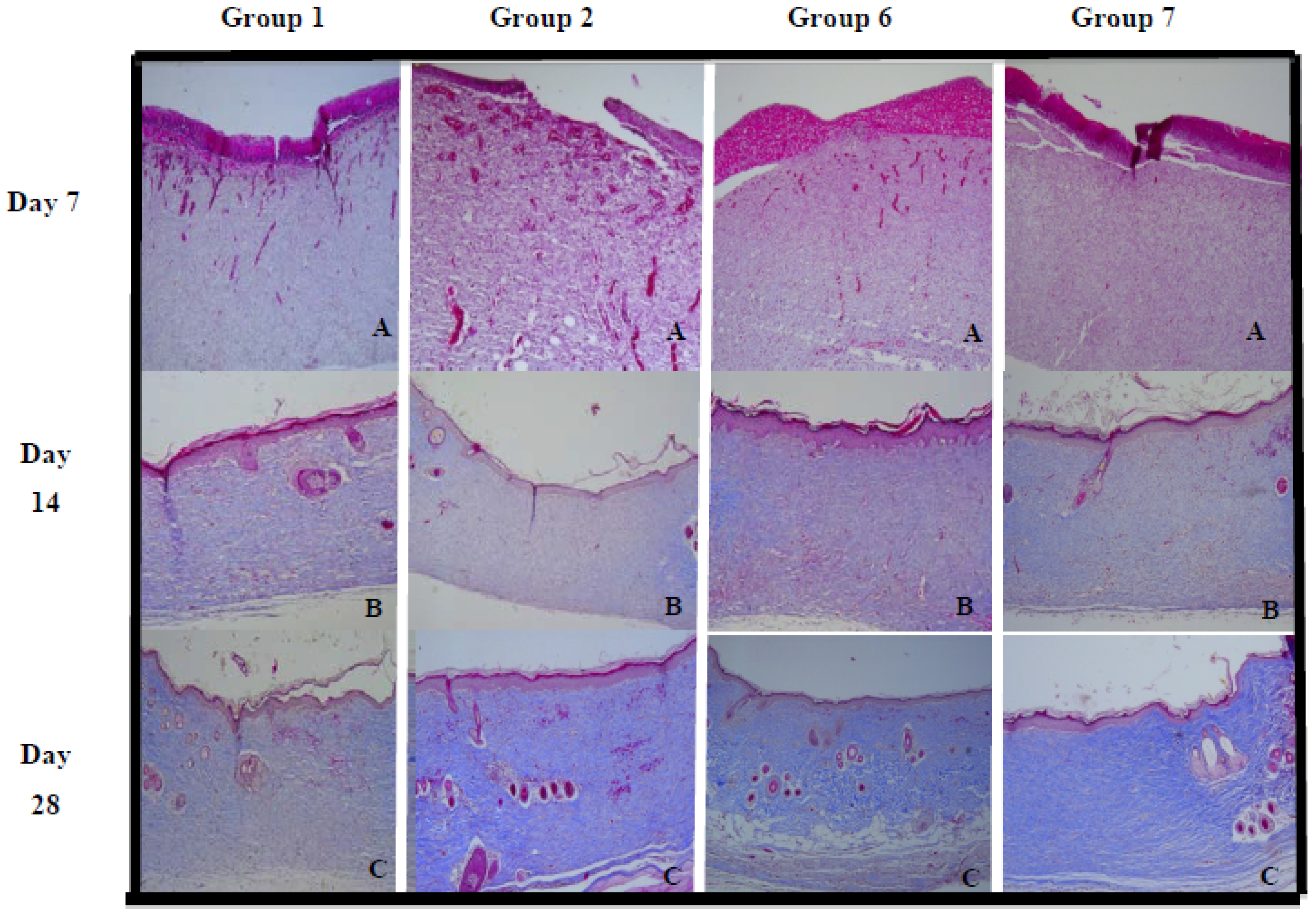
| Group | Day 7 (%) | Day 14 (%) | Day 21 (%) | Day 28 (%) |
|---|---|---|---|---|
| 1 | 22 ± 0.96 | 26.77 ± 1.27 | 61.84 ± 0.94 | 78.06 ± 1.22 |
| 2 | 20 ± 0.62 | 36.2 ± 1.36 | 65.63 ± 0.87 | 81.56 ± 1.04 |
| 3 | 20.6 ± 0.34 | 39.62 ± 0.29 | 67.76 ± 0.85 | 88.54 ± 1.34 |
| 4 | 14.87 ± 0.44 | 38.18 ± 0.69 | 67.15 ± 2.12 | 81.08 ± 1.09 |
| 5 | 19.18 ± 0.54 | 30.32 ± 2.33 | 63.23 ± 0.76 | 83.78 ± 1.24 |
| 6 | 19.75 ± 0.34 | 35.51 ± 1.44 | 69.41 ± 0.24 | 91.78 ± 1.02 |
| 7 | 21.08 ± 0.53 | 27.08 ± 1.03 | 60.05 ± 0.72 | 86.21 ± 1.12 |
3. Experimental
3.1. Plant Material
3.2. Preparation of Extract and Isolation of Carbazole Alkaloids
3.3. Extraction of Essential Oil and Analysis
3.4. Wound Healing Activity
3.4.1. Experimental Animals
3.4.2. Grouping of Animals
3.4.3. Excision Wound Model and Treatment of Wounds
3.4.4. Wound Contraction and Epithelialization Time
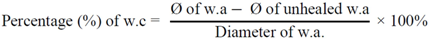
3.4.5. Histopathological Evaluation
3.4.6. Computerized Collagen Density Evaluation
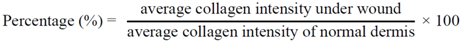
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Adetutu, A.; Morgan, W.A.; Corcoran, O. Ethnopharmacological survey and in vitro evaluation of wound-healing plants used on South-western Nigeria. J. Ethnopharmacol. 2011, 137, 50–56. [Google Scholar] [CrossRef]
- Gurung, S.; Basnet, N.S. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J. Ethanopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Suntar, I.; Akkol, E.K.; Keles, H.; Oktem, A.; Baser, K.H.C.; Yesilada, E. A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J. Ethanopharmacol. 2011, 134, 89–96. [Google Scholar] [CrossRef]
- Chah, K.F.; Eze, C.A.; Esimone, C.O. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006, 104, 164–167. [Google Scholar] [CrossRef]
- Muthusamy, S.K.; Ramasamy, S.; Harinarayan, V.R.; Praveen, K.S. Wound healing potential of Cassia fistula on infected albino rat model. J. Surg. Res. 2006, 131, 283–298. [Google Scholar] [CrossRef]
- Balekar, N.; Katkam, N.G.; Nakpheng, T.; Jehtae, K.; Srichana, T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J. Ethanopharmacol. 2012, 141, 817–824. [Google Scholar] [CrossRef]
- Kumar, B.; Viayakumar, M.; Govindarajan, G.; Pushpangadan, P. Ethanopharmacological approaches to wound healing-Exploring medicinal plants of India. J. Ethanopharmacol. 2007, 114, 103–113. [Google Scholar] [CrossRef]
- Shailajan, S.; Menon, S.; Pednekar, S.; Singh, A. Wound healing efficacy of Jatyadi Taila: In vivo evaluation in rat using excision wound model. J. Ethanopharmacol. 2011, 138, 99–104. [Google Scholar] [CrossRef]
- Basal, A.A.M. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J. Ethanopharmacol. 2010, 131, 443–450. [Google Scholar] [CrossRef]
- Annan, K.; Houghton, P.J. Antibacterial, antioxidant and fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq. and Gossypium arboreum L., wound-healing plants of Ghana. J. Pharmacol. 2008, 119, 141–144. [Google Scholar]
- Csupor, D.; Blazso, G.; Balogh, A.; Hohmann, J. The traditional Hungarian medicinal plant Centaureas adleriana Janka accelerates wound healing in rats. J. Ethnopharmacol. 2010, 127, 193–195. [Google Scholar] [CrossRef]
- Maldini, M.; Sosa, S.; Montoro, P.; Giangaspero, A.; Balick, M.J.; Pizza, C.; Loggia, R.D. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Brysonimacrassi folia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J. Ethanopharmacol. 2009, 122, 430–433. [Google Scholar] [CrossRef]
- Khanum, F.; Anilakumar, K.R.; Krishna, K.R.S.; Viswanathan, K.R.; Santhanam, K. Anticarcinogenic effects of curry leaves in dimethylhydrazine-treated rats. Plant Foods Hum. Nutr. 2000, 55, 347–355. [Google Scholar] [CrossRef]
- Raina, V.K.; Lal, R.K.; Tripathi, S.; Khan, M.; Syamasundar, K.V.; Srivastava, S.K. Essential oil composition of genetically diverse stocks of Murraya koenigii from India. Flavour Fragr. J. 2002, 17, 144–146. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Ayoola, O.F.; Iwalewa, E.O.; Akindahunsi, A.A.; Omisore, N.O.A.; Adewunmi, C.O.; Adenowo, T.K. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef]
- Arulselvan, P.; Subramanian, S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem. Biol. Interact. 2007, 165, 155–164. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Bhuiyan, M.N.I.; Yusuf, M. Chemical composition of the essential oil of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Banglad. J. Pharmacol. 2008, 3, 59–63. [Google Scholar]
- Bhattacharjee, S.K. Handbook of Medicinal Plants, 5th Revised & Enlarged; Pointer Publishers: New Delhi, India, 2008. [Google Scholar]
- Bhattacharya, K.; Samanta, S.K.; Tripathi, R.; Mallick, A.; Chandra, S.; Pal, B.C.; Shaha, C.; Mandal, C. Apototic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem. Pharmacol. 2010, 79, 361–372. [Google Scholar]
- Mandal, S.; Nayak, A.; Kar, M.; Banerjee, S.K.; Das, A.; Upadhyay, S.N.; Singh, R.K.; Banerji, A.; Banerji, J. Antidiarrhoel activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 2012, 81, 72–74. [Google Scholar]
- Tang, T.; Yin, L.W.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Silva, K.A.B.S.; Manjavachi, M.N.; Paszcuk, A.F.; Pivatto, M.; Bolzani, V.S.; Calixto, J.B. Plant derived alkaloids (−)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology 2012, 62, 967–977. [Google Scholar] [CrossRef]
- Dasgupta, T.; Rao, A.R.; Yadava, P.K. Chemomodulatory action of curry leaf (Murraya koenigii) extract on hepatic and extrahepatic xenobiotic metabolizing enzymes, antioxidant levels, lipid peroxidation, skin and forestomachpapillomagenes. Nutr. Res. 2003, 23, 1427–1446. [Google Scholar] [CrossRef]
- Kaushik, G.; Satya, S.; Khandelwal, R.K.; Naik, S.N. Commonly consumed Indian plant food materials in the management of diabetes mellitus. Diabetes Metab. Syndr. 2010, 4, 21–40. [Google Scholar] [CrossRef]
- Nayak, B.S.; Anderson, M.; Pereira Pinto, L.M. Evaluation of wound-healing potential of Catharanthusroseus leaf extract in rats. Fitoterapia 2007, 78, 540–544. [Google Scholar] [CrossRef]
- Deshmukh, P.T.; Fernandes, J.; Atul, A.; Toppo, E. Wound healing activity of Calotropis gigantean root bark in rats. J. Ethanopharmacol. 2009, 125, 178–181. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L.; Nitiss, J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999, 47, 444–447. [Google Scholar] [CrossRef]
- Ningappa, M.B.; Dinesha, R.; Srinivas, L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem. 2008, 106, 720–728. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kikuzaki, H.; Lajis, N.; Nakatani, N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. [Google Scholar] [CrossRef]
- Ningappa, M.B.; Dhananjaya, B.L.; Dinesha, R.; Harsha, R.; Srinivas, L. Potent antibacterial property of APC protein from curry leaves (Murraya koenigii L.). Food Chem. 2010, 118, 747–750. [Google Scholar] [CrossRef]
- Nagappan, T.; Ramasamy, P.; Abdul Wahid, M.E.; Chandrasegaran, T.; Vairappan, C.S. Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules 2011, 16, 9651–9664. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nagappan, T.; Segaran, T.C.; Wahid, M.E.A.; Ramasamy, P.; Vairappan, C.S. Efficacy of Carbazole Alkaloids, Essential Oil and Extract of Murraya koenigii in Enhancing Subcutaneous Wound Healing in Rats. Molecules 2012, 17, 14449-14463. https://doi.org/10.3390/molecules171214449
Nagappan T, Segaran TC, Wahid MEA, Ramasamy P, Vairappan CS. Efficacy of Carbazole Alkaloids, Essential Oil and Extract of Murraya koenigii in Enhancing Subcutaneous Wound Healing in Rats. Molecules. 2012; 17(12):14449-14463. https://doi.org/10.3390/molecules171214449
Chicago/Turabian StyleNagappan, Thilahgavani, Thirukanthan Chandra Segaran, Mohd Effendy Abdul Wahid, Perumal Ramasamy, and Charles S. Vairappan. 2012. "Efficacy of Carbazole Alkaloids, Essential Oil and Extract of Murraya koenigii in Enhancing Subcutaneous Wound Healing in Rats" Molecules 17, no. 12: 14449-14463. https://doi.org/10.3390/molecules171214449
APA StyleNagappan, T., Segaran, T. C., Wahid, M. E. A., Ramasamy, P., & Vairappan, C. S. (2012). Efficacy of Carbazole Alkaloids, Essential Oil and Extract of Murraya koenigii in Enhancing Subcutaneous Wound Healing in Rats. Molecules, 17(12), 14449-14463. https://doi.org/10.3390/molecules171214449





