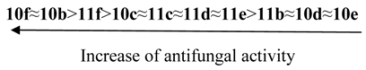3. Experimental
3.1. General
The 1H- and 13C-NMR spectra were recorded on Varian Unity Inova (300 MHz, 75 MHz) spectrometer operating on Fourier transform mode, using DMSO-d6 and CDCl3 as solvents and TMS as an internal standart (chemical shifts in δ, ppm). IR spectra (ν, cm−1) were recorded on a Perkin Elmer Spectrum BX FT–IR spectrometer, KBr tablets. Mass spectra were obtained on Waters ZQ 2000 spectrometer using the atmospheric pressure chemical ionization (APCI) mode and operating at 25 V. Elemental analyses were performed on CE-440 elemental analyzer. Melting points were determined on automatic APA1 melting point apparatus and are uncorrected. TLC was performed on Merck, Silica gel 60 F254 (Kieselgel 60 F254) silica gel plates.
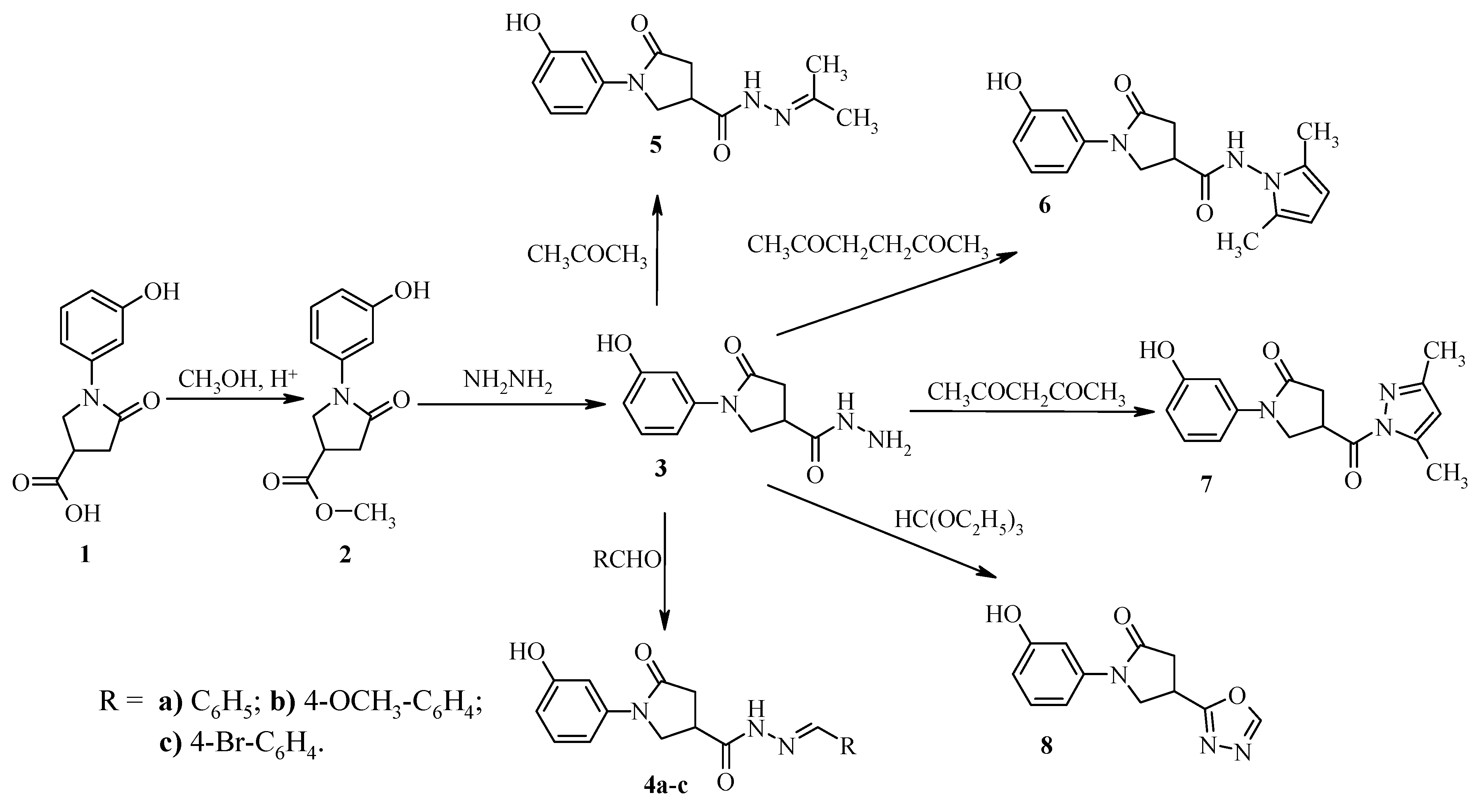
Methyl 1-(3-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylate (2). A mixture of 5-oxopyrrolidine 1 (17.7 g, 0.08 mol), methanol (100 mL) and catalytic amount of conc. H2SO4 was refluxed for 5 h. Then the solvent was evaporated. The precipitated product was neutralized with 5% sodium bicarbonate solution and filtered off, washed with 2-propanol. The crude product was recrystallized from 2-propanol. White crystals, (15.9 g, 85%), m.p. 188–189 °C; νmax/cm−1 1660 and 1740 (C=O) and 3159 (OH). 1H-NMR (DMSO-d6): δH 2.65–2.86 (2H, m, CH2CO), 3.38–3.49 (1H, m, CH), 3.67 (3H, s, CH3), 3.88–4.06 (2H, m, NCH2), 6.52–7.28 (4H, m, HAr), 9.50 (1H, s, OH). 13C-NMR (DMSO-d6) δC 34.8 (CH), 35.1 (CH2CO), 49.8 (CH2N), 52.1 (CH3), 106.7, 109.8, 111.3, 129.4, 140.0, 157.5 (CAr), 171.4, 173.1 (C=O). MS, m/z, (%) = 258 ([M+Na]+ 100); Anal. Calcd for C12H13NO4 (235.24): C, 61.27; H, 5.57; N, 5.95. Found: C, 61.34; H, 5.51; N, 5.89.
1-(3-Hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (3). A mixture of ester 2 (11.0 g, 0.047 mol), 2-propanol (100 mL) and 98% hydrazine hydrate (8.0 g, 0.16 mol) was refluxed for 5 h. The reaction mixture was cooled, the precipitate was filtered off, washed with 2-propanol and purified by recrystallization from water. White crystals, (10.1 g, 92%), m.p. 208–209 °C; νmax/cm−1 1697 and 1660 (C=O), 3131 (OH), 3314 and 3253 (NH, NH2). 1H-NMR (DMSO-d6): δH 2.55–2.75 (2H, m, CH2CO), 3.07–3.19 (1H, m, CH), 3.74–3.98 (2H, m, NCH2), 4.29 (2H, br. s, NH2), 6.52–7.26 (4H, m, HAr), 9.27 (1H, s, NH), 9.48 (1H, s, OH). 13C-NMR (DMSO-d6) δC 33.9 (CH), 35.8 (CH2CO), 50.7 (CH2N), 106.6, 109.7, 111.2, 129.4, 140.2, 157.5 (CAr), 171.6, 171.9 (C=O). MS, m/z, (%) = 258 ([M+Na]+ 100); Anal. Calcd for C11H13N3O3 (235.24): C, 56.16; H, 5.57; N, 17.86. Found: C, 56.22; H, 5.32; N, 17.84.
3.2. General Synthetic Procedure for the Synthesis of Hydrazones 4a–c
A mixture of hydrazide 3 (3.5 g, 0.015mol), the corresponding aldehyde (0.016 mol) and 2-propanol (30 mL) was refluxed for 3 h. The reaction mixture was cooled, the precipitate was filtered off and washed with 2-propanol.
1-(3-Hydroxyphenyl)-5-oxo-N'-(phenylmethylene)pyrrolidine-3-carbohydrazide (4a). White crystals, (4.36 g, 91%), m.p. 194–195 °C; νmax/ cm−1 1590 (C=N), 1664 and 1693 (C=O), 3225 (OH) and 3334 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 40/60) 2.68–2.89 (2H, m, CH2CO), 3.27–3.38 (1H, m, CH), 3.89–4.14 (2H, m, NCH2), 6.52–7.77 (9H, m, HAr), 8.04, 8.22 (1H, 2s, NCH), 9.47 (1H, s, OH), 11.57, 11.63 (1H, 2s, NH). 13C-NMR (DMSO-d6) δC 32.7, 34.7, 35.0, 32.7 (CH, CH2CO), 50.1, 50.5 (CH2N), 106.6, 109.8, 111.1, 126.8, 126.9, 128.8, 129.3, 129.8, 130.0, 134.0, 140.1, 140.2, 143.6, 146.9, 157.5, 168.6, (CAr, NCH), 171.7, 171.9, 173.5 (C=O). MS, m/z, (%) = 346 ([M+Na]+ 100); Anal. Calcd for C18H17N3O3 (323.35): C, 66.86; H, 5.30; N, 13.00. Found: C, 66.75; H, 5.24; N, 13.08.
1-(3-Hydroxyphenyl)-N'-[(4-methoxyphenyl)methylene]-5-oxopyrrolidine-3-carbohydrazide (4b). White crystals, (4.68 g, 89%), m.p. 200–201 °C (from dioxane); νmax/cm−1 1600 (C=N), 1609 and 1661, (C=O), 3088 (OH) and 3231 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 40/60) 2.65–2.85 (2H, m, CH2CO), 3.24–3.37 (1H, m, CH), 3.85–4.14 (2H, m, NCH2), 6.51–7.69 (8H, m, HAr), 7.98, 8.16 (1H, 2s, NCH), 9.47 (1H, s, OH), 11.44, 11.50 (1H, 2s, NH). 13C-NMR (DMSO-d6) δC 32.7, 34.7, 35.0, 35.8 (CH, CH2CO), 50.1, 50.6 (CH2N), 55.2 (OCH3), 106.6, 109.8, 111.1, 114.3, 126.7, 128.4, 128.6, 129.3, 140.2, 143.4, 146.8, 157.5, 160.6, 160.8, 168.4 (CAr, N=CH), 171.8, 171.9, 173.2 (C=O). MS, m/z, (%) = 376,6 ([M+Na]+ 100); Anal. Calcd for C19H19N3O4 (353.38): C, 64.58; H, 5.42; N, 11.89. Found: C, 64.42; H, 5.39; N, 11.79.
1-(3-Hydroxyphenyl)-N'-[(4-bromophenyl)methylene]-5-oxopyrrolidine-3-carbohydrazide (4c). White crystals, (5.27 g, 88%), m.p. 255–256 °C (from dioxane); νmax/cm−1 1599 (C=N), 1663 and 1698 (C=O), 2973 (OH) and 3172 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 40/60) 2.64–2.89 (2H, m, CH2CO), 3.26–3.40 (1H, m, CH), 3.85–4.18 (2H, m, NCH2), 6.49–7.70 (8H, m, HAr), 8.01, 8.19 (1H, 2s, NCH), 9.47 (1H, s, OH), 11.63, 11.71 (1H, 2s, NH). 13C-NMR (DMSO-d6) δC 32.7, 34.7, 34.9, 35.7 (CH, CH2CO), 50.0, 50.5 (CH2N), 106.6, 109.8, 111.1, 123.0, 123.3, 128.7, 128.9, 129.3, 131.7, 133.4, 140.1, 140.2, 142.4, 145.7, 157.5 (CAr), 171.6, 171.9 173.6 (C=O). MS, m/z, (%) = 403 ([M+H]+ 95), 405 ([M+H+2]+ 100). Anal. Calcd for C18H16BrN3O3 (402.25): C, 53.75; H, 4.01; N, 10.45. Found: C, 53.31; H, 4.19; N, 10.71.
1-(3-Hydroxyphenyl)-N'-isopropylidene-5-oxopyrrolidine-3-carbohydrazide (5). A mixture of compound 3 (1.0 g 4.3 mmol) and dry 2-propanone (30 mL) was refluxed for 4 h. The solvent was evaporated under vacuum, and the precipitated product was filtered off, washed with ethyl ether and purified by recrystallization from 2-propanol. White crystals, (0.8 g, 68%), m.p. 185–186 °C; IR νmax/cm−1 1597 (C=N), 1675, 1659 (C=O), 3180 (OH) and 3235 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 55/45)1.87, 1.88 (3H, 2s, CH3), 1.93 (3H, s, CH3), 2.59–2.80 (2H, m, CH2CO), 3.34–3,47 (1H, m, CH), 3.77–4.06 (2H, m, NCH2), 6.51–7.29 (4H, m, HAr), 9.46 (1H, s, OH), 10.21, 10.30 (1H, 2s, NH). 13C-NMR (DMSO-d6) δC 17.1, 17.6, 24.9, 25.2 (2CH3), 32.8, 34.2, 35.1, 35.9 (CH, CH2CO), 50.2, 50.9 (CH2N), 106.6, 106.6, 109.7, 109.8, 111.1, 129.3, 140.2, 140.3, 151.2, 156.1, 157.4, 168.6 (CAr), 171.9, 172.0, 173.6 (C=O). MS, m/z, (%) = 298.6 ([M+Na]+ 100); Anal. Calcd for C14H17N3O3 (275.31): C, 61.08; H, 6.22; N, 15.26. Found: C, 60.98; H, 6.31; N, 15.30.
N-(2,5-Dimethyl-1H-pyrrol-1-yl)-1-(3-hydroxyphenyl)-5-oxopyrrolidine-3-carboxamide (6). To a solution of hydrazide 3 (3.0 g, 0.013 mol) in 2-propanol (40 mL) 2,5-hexanedione (4.6 g, 0.04 mol) and glacial acetic acid (3 mL) were added. The reaction mixture was stirred and refluxed for 6 h. Then it was cooled to room temperature. The precipitated product was filtered off, washed with ethyl ether and recrystallized from 2-propanol. White crystals, (2.8 g, 70%), m.p. 170–171 °C; νmax/cm−1 1603, 1676 (C=O), 3046 (OH) and 3247 (NH). 1H-NMR (DMSO-d6): δH 1.99 (6H, s, 2CH3), 2.67–2.94 (2H, m, COCH2), 3.39–3.51 (1H, m, CH), 3.88–4.15 (2H, m, NCH2), 5.65 (2H, 2, 2CH), 6.53–7.28 (m, 4H, HAr), 9.51 (1H, s, OH), 10.92 (1H, s, NH). 13C-NMR (DMSO-d6) δC 10.8 (2CH3), 33.9 (CH), 35.7 (CH2CO), 50.4 (CH2N), 103.0, 106.7, 109.9, 111.3, 126.7, 129.4, 140.1, 157.5 (Cpyrrole, CAr), 171.4, 171.8 (2C=O). MS, m/z, (%) = 336 ([M+Na]+ 100); Anal. Calcd for C17H19N3O3 (313.36): C, 65.16; H, 6.11; N, 13.41. Found: C, 65.22; H, 6.06; N, 13.45.
4-[(3,5-Dimethyl-1H-pyrazol-1-yl)carbonyl]-1-(3-hydroxyphenyl)pyrrolidin-2-one (7). A mixture of hydrazide 3 (3.0 g, 0.013 mol), 2,4-pentanedione (4.0 g, 0.04 mol), 2-propanol (30 mL) and a catalytic amount of hydrochloric acid was refluxed for 6 h. The solvent was evaporated under vacuum to dryness, and the oily product was triturated with ethyl ether. The obtained solid was filtered off and washed with ethyl ether. The crude product was purified by recrystallization from 2-propanol. White crystals, (2.0 g, 53%), m.p. 165–166 °C; νmax/cm−1 1601 (C=N), 1663, 1722, (C=O) and 3148 (OH). 1H-NMR (DMSO-d6): δH 2.21, (3H, s, CH3), 2.48 (3H, s, CH3), 2.76–2.94 (2H, m, COCH2), 3.94–4.20 (2H, m, NCH2), 4.39–4.51 (1H, m, CH), 6.23(1H, s, CH), 6.52–7.25 (4H, m, HAr), 9.49 (1H, s, OH). 13C-NMR (DMSO-d6) δC 13.5, 13.9 (2CH3), 35.2 (CH), 35.2 (CH2CO), 50.1 (CH2N), 106.7, 109.9, 111.2, 111.5, 129.3, 139.9, 143.8, 152.0, 157.4 (Cpyrazole, CAr), 171.4, 171.5 (C=O). MS, m/z, (%) = 322 ([M+Na]+ 100); Anal. Calcd for C16H17N3O3 (299.34): C, 64.20; H, 5.72; N, 14.04. Found: C, 64.31; H, 5.75; N, 14.08.
1-(3-Hydroxyphenyl)-4-[1,3,4]oxadiazol-2-yl-pyrrolidin-2-one (8). A mixture of compound 3 (7.0 g, 0.03 mol), triethyl orthoformate (35.6 g, 0.24 mol) and p-toluenesulfonic acid (1.14 g, 6 mmol) was refluxed for 8 h. After cooling, the precipitate was filtered off, washed with ethyl ether and purified by recrystallization from 2-propanol. White crystals, (3.32 g, 46%), m.p. 181–182 °C; νmax/cm−1 1600 (C=N), 1679 (C=O) and 3184 (OH). 1H-NMR (DMSO-d6): δH 2.81–3.12 (2H, m, CH2CO), 4.02–4.31 (3H, m, NCH2, CH), 6.52–7.27 (4H, m, HAr), 9.22 (1H, s, N=CH), 9.48 (1H, s, OH); 13C-NMR (DMSO-d6) δC 27.5 (CH), 35.9 (CH2CO), 50.6 (CH2N), 106.9, 109.9, 111.4, 129.3, 139.8, 154.7, 157.4, 166.4 (Coxadiazole, CAr), 170.9 (C=O); MS, m/z, (%) = 268 ([M+Na]+ 100); Anal. Calcd for C12H11N3O3 (245.24): C, 58.77; H, 4.52; N, 17.13. Found: C, 58.59; H, 4.41; N, 17.19.
3.3. General Synthetic Procedure for the Synthesis of Compounds 10b–f
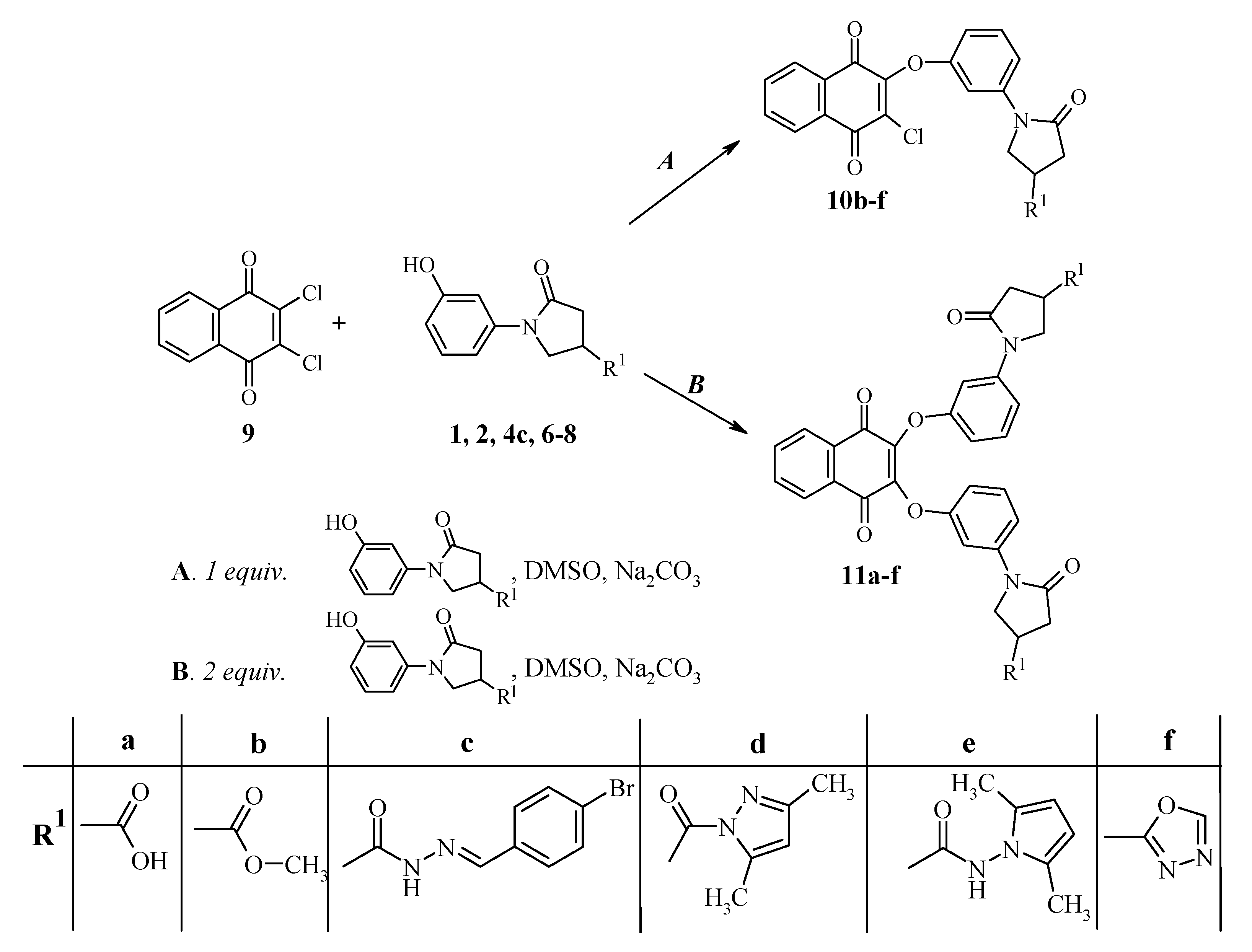
A mixture of 2,3-dichloro-1,4-naphthoquinone 9 (0.88 g 3.9 mmol), the corresponding 1-(3-hydroxyphenyl)-5-oxopyrrolidine derivative 2, 4c, 6–8 (3.9 mmol), sodium carbonate (1.0 g) in dimethyl sulfoxide (15 mL) was stirred at room temperature for 24 h. The reaction mixture was diluted with water (70 mL), the precipitate was filtered off and washed with water.
Methyl 1-{3-[(3-chloro-1,4-dioxo-1,4-dihydro-2-naphthalenyl)oxy]phenyl}-5-oxo-3-pyrrolidinecarboxylate (10b). The crude product was purified by chromatography on a silica gel 60 column (2-propanone-hexane, 1:1), Rf 0.56. Yellow crystals, (1.0 g, 61%), m.p. 144–145 °C; νmax/cm−1 1663, 1679, 1692 and 1736 (C=O). 1H-NMR (DMSO-d6): δH 2.66–2.86 (2H, m, COCH2), 3.38–3.50 (1H, m, CH), 3.66 (3H, s, CH3), 3.91–4.11 (2H, m, NCH2), 6.92–8.15 (8H, m, HAr). 13C-NMR (DMSO-d6) δC 34.8 (CH), 35.1 (CH2CO), 49.7 (CH2N), 52.1 (OCH3), 107.3, 111.6, 114.3, 126.5, 126.6, 129.7, 130.5, 131.3, 133.5, 134.4, 134.6, 140.3, 152.3, 156.3 (CAr), 171.8, 172.9, 177.7, 178.1 (C=O). MS, m/z, (%) = 449 ([M+Na]+ 100), 451 ([M+Na+2]+ 35); Anal. Calcd for C22H16ClNO6 (425.83): C, 62.05; H, 3.79; N, 3.29. Found: C, 62.10; H, 3.88; N, 3.18.
N'-[(4-Bromophenyl)methylidene]-1-{3-[(3-chloro-1,4-dioxo-1,4-dihydro-2-naphthalenyl)oxy]phenyl}-5-oxo-3-pyrrolidinecarbohydrazide (10c). The crude product was purified by chromatography on a silica gel 60 column (ethyl acetate–hexane, 1:1), Rf 0.63. Yellow crystals, (2.1 g, 90%), m.p. 185–186 °C; νmax/cm−1 1594 (C=N), 1630, 1676, 1690, 1725 (C=O) and 3215 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 40/60) 2.68–2.91 (2H, m, COCH2), 3.30–3.39 (1H, m, CH), 3.93–4.17 (2H, m, NCH2), 6.93–8.20 (13H, m, N=CH, HAr), 11.65, 11.71 (1H, 2s, NH). 13C-NMR (DMSO-d6) δC 32.7, 34.7, 35.0, 35.7 (CH, CH2CO), 49.9, 50.4 (CH2N), 107.2, 107.4, 111.5, 114.2, 114.3, 123.0, 123.3, 126.4, 126,.6, 128.7, 128.8, 129.7, 130.5, 131.2, 131.7, 133.3, 133.4, 134.4, 134.5, 140.4, 142.4, 145.8, 152.3, 156.3, 168.6 (CAr), 172.1, 172.3, 173.4, 177.7, 178.0 (C=O). Anal. Calcd for C28H19BrClN3O5 (592.82): C, 56.73; H, 3.23; N, 7.09. Found: C, 56.85; H, 3.36; N, 6.98.
2-Chloro-3-{3-[4-(3,5-dimethylpyrazole-1-carbonyl)-2-oxopyrrolidin-1-yl]phenoxy}-[1,4]naphthoquinone (10d). The crude product was recrystallized from ethanol. Yellow crystals, (1.4 g, 72%), m.p. 130–131 °C; νmax/cm−1 1572 (C=N), 1667, 1673, 1698 and 1735 (C=O). 1H-NMR (DMSO-d6): δH 2.19 (3H, s, CH3), 2.47 (3H, s, CH3), 2.77–2.96 (2H, m, COCH2), 3.97–4.24 (2H, m, NCH2), 4.40–4.52 (1H, m, CH), 6.21, 6.22 (1H, 2s, C=CH), 6.94–8.15 (8H, m, HAr). 13C-NMR (DMSO-d6) δC 13.5, 14.0 (2CH3), 35.2 (CH), 35.5 (CH2CO), 50.2 (CH2N), 107.4, 111.6, 114.4, 126.5, 126.6, 129.7, 130.5, 131.3, 133.4, 134.4, 134.6, 140.3, 143.8, 152.1, 152.3, 156.3 (Cpyrazole, CAr), 171.8, 172.5, 177.7, 178.1 (C=O). MS, m/z, (%) = 513 ([M+Na]+ 100); 515 ([M+Na+2]+ 35) Anal. Calcd for C26H20ClN3O5 (489.92): C, 63.74; H, 4.11; N, 8.58. Found: C, 63.65; H, 3.98; N, 8.49.
1-{3-[(3-Chloro-1,4-dioxo-1,4-dihydro-2-naphthalenyl)oxy]phenyl}-N-(2,5-dimethyl-1H-pyrrol-1-yl)-5-oxo-3-pyrrolidinecarboxamide (10e). The crude product was recrystallized from ethanol. Yellow crystals, (1.8 g, 90%), m.p. 181–182 °C; νmax/cm−1 1578 (C=N), 1650, 1676, 1696, 1710 (C=O) and 3313 (NH). 1H-NMR (DMSO-d6): δH 1.97 (3H, s, CH3), 1.99 (3H, s, CH3), 2.68–2.96 (2H, m, COCH2), 3.40–3.52 (1H, m, CH), 3.94–4.17 (2H, m, NCH2), 5.64 (2H, s, 2CH), 6.94–8.16 (8H, m, HAr), 10.88 (1H, s, NH); 13C-NMR (DMSO-d6) δC 10.8 (2CH3), 33.8 (CH), 35.7 (CH2CO), 50.2 (CH2N), 102.9, 107.4, 111.6, 114.2, 126.4, 126.6, 129.7, 130.5, 131.2, 133.3, 134.4, 134.5, 140.3, 152.3, 156.2 (Cpyrrole, CAr), 171.6, 171.7, 177.6, 177.9 (C=O). MS, m/z, (%) = 527 ([M+Na]+ 100); 529 ([M+Na+2]+ 35)Anal. Calcd for C27H22ClN3O5 (503.95): C, 64.35; H, 4.40; N, 8.34. Found: C, 64.22; H, 4.39; N, 8.40.
2-Chloro-3-[3-(4-[1,3,4]oxadiazol-2-yl-2-oxopyrrolidin-1-yl)phenoxy]-[1,4]naphthoquinone (10f). The crude product was purified by chromatography on a silica gel 60 column (2-propanone-hexane, 1:1), Rf 0.29. Yellow crystals, (1.4 g, 82%), m.p. 195–196 °C; νmax/cm−1 1576, 1597 (C=N), 1678, 1698 and 1743 (C=O). 1H-NMR (DMSO-d6): δH 2.84–3.12 (2H, m, COCH2), 4.06–4.34 (3H, m, CH, NCH2), 6.96–8.15 (8H, m, HAr), 9.23 (1H, s, N=CH). 13C-NMR (DMSO-d6) δC 27.5 (CH), 36.0 (CH2CO), 50.6 (CH2N), 107.4, 111.7, 114.5, 126.5, 126.6, 129.8, 130.5, 131.3, 133.5, 134.4, 134.6, 140.2, 152.3, 154.9, 156.3, 166.3 (CAr, Coxadiazole), 171.4, 177.7, 178.1 (C=O). MS, m/z, (%) = 459 ([M+Na]+ 100); 461 ([M+Na+2]+ 35) Anal. Calcd for C22H14ClN3O5 (435.83): C, 60.63; H, 3.24; N, 9.64. Found: C, 60.51; H, 3.31; N, 9.69.
3.4. General Synthetic Procedure for the Synthesis of Compounds 11a–f
A mixture of 2,3-dichloro-1,4-naphthoquinone 9 (3.3 mmol, 0.75 g), the corresponding 1-(3-hydroxyphenyl)-5-oxopyrrolidine derivative 1, 2, 5–7, 8c (6.6 mmol), sodium carbonate (2.0 g) and dimethyl sulfoxide (15 mL) was stirred at room temperature for 24 h. The reaction mixture was diluted with water (70 mL), the precipitate was filtered off and washed with water.
1-[3-({3-[3-(4-Carboxy-2-oxo-1-pyrrolidinyl)phenoxy]-1,4-dioxo-1,4-dihydro-2-naphthalenyl}oxy)phenyl]-5-oxo-3-pyrrolidinecarboxylic acid (11a). The crude product was purified by dissolving them in sodium hydroxide solution (5%), filtering the solution, and acidifying the filtrate with acetic acid up to pH 6. Orange crystals, (1.4 g, 72%), m.p. 170–171 °C; νmax/cm−1 1671, 1700, 1730, (C=O) and 3072 (OH). 1H-NMR (DMSO-d6): δH 2.58–2.76 (4H, m, 2COCH2), 3.10–3.23 (2H, m, 2CH), 3.80–3.97 (4H, m, 2NCH2), 6.84–8.07 (12H, m, HAr). 13C-NMR (DMSO-d6) δC 35.7 (CH), 35.9 (CH2CO), 50.7 (CH2N), 107.1, 107.3, 111.7, 113.9, 126.0, 129.3, 130.8, 134.3, 140.2, 145.3, 145.4, 156.4, 156.4 (CAr), 172.6, 174.8, 179.9 (C=O). MS, m/z, (%) = 619 ([M+Na]+ 100); Anal. Calcd for C32H24N2O10 (596.56): C, 64.43; H, 4.06; N, 4.70. Found: C, 64.39; H, 3.98; N, 4.66.
Methyl 1-{3-[(3-{3-[4-(methoxycarbonyl)-2-oxo-1-pyrrolidinyl]phenoxy}-1,4-dioxo-1,4-dihydro-2-naphthalenyl)oxy]phenyl}-5-oxo-3-pyrrolidinecarboxylate (11b). The crude product was chromatographed over a silica gel 60 column (2-propanone-hexane, 1:1), Rf 0.29. Orange crystals, (0.9 g, 45%), m.p. 90–91 °C; νmax/cm−1 1676, 1703 and 1737 (C=O). 1H-NMR (DMSO-d6): δH 2.64–2.83 (4H, m, 2COCH2), 3.35–3.48 (2H, m, 2CH), 3.66 (6H, s, 2CH3), 3.84–4.04 (4H, m, 2NCH2), 6.88–8.07 (12H, m, HAr). 13C-NMR (DMSO-d6) δC 34.7 (2CH), 34.9 (2CH2CO), 49.7 (2CH2N), 52.1 (2OCH3), 107.3, 111.8, 113.9, 126.0, 129.4, 130.8, 134.3, 139.9, 145.6, 156.6 (CAr), 171.6, 172.9, 179.9 (C=O). MS, m/z, (%) = 647 ([M+Na]+ 100); Anal. Calcd for C34H28N2O10 (624.61): C, 65.38; H, 4.52; N, 4.48. Found: C, 65.44; H, 4.49; N, 4.52.
N'-[(4-Bromophenyl)methylidene]-1-{3-[(3-{3-[4-({2-[(4-bromophenyl)methylidene]hydrazino}carbonyl)-2-oxo-1-pyrrolidinyl]phenoxy}-1,4-dioxo-1,4-dihydro-2-naphthalenyl)oxy]phenyl}-5-oxo-3-pyrrolid-inecarbohydrazide (11c). The crude product was chromatographed over a silica gel 60 column (ethyl acetate–methanol, 11:1), Rf 0.58. Yellow crystals, (1.2 g, 38%), m.p. 189–190 °C; νmax/cm−1 1591 (C=N), 1678, 1693, 1731 (CO) and 3209 (NH). 1H-NMR (DMSO-d6): δH (E/Z, 40/60) 2.69–2.83 (4H, m, 2COCH2), 3.23–3.39 (2H, m, 2CH), 3.84–4.11 (4H, m, 2NCH2), 6.86–8.19 (22H, m, 2N=CH, HAr), 11.63, 11.69 (2H, 2s, 2NH). 13C-NMR (DMSO-d6) δC 32.6, 34.6, 35.0, 35.7, (2CH, 2CH2CO), 49.9, 50.4 (2CH2N), 107.3, 111.6, 114.0, 123.0, 123.2, 126.0, 128.7, 128.8, 129.3, 130.8, 131.7, 133.3, 134.3, 140.1, 142.4, 145.7, 156.6 (2N=CH, CAr), 172.1, 173.4, 179.9 (C=O). Anal. Calcd for C46H34Br2N6O8 (958.63): C, 57.64; H, 3.58; N, 8.77. Found: C, 57.51; H, 3.39; N, 8.69.
2,3-Bis(3-{4-[(3,5-dimethyl-1H-pyrazol-1-yl)carbonyl]-2-oxo-1-pyrrolidinyl}phenoxy)naphthoquinone (11d). The crude product was chromatographed over a silica gel 60 column (2-propanone-hexane, 1:1), Rf 0.72. Yellow crystals, (1.1 g, 43%), m.p. 136–137 °C; νmax/cm−1 1587 (C=N), 1677, 1706 and 1723 (C=O). 1H-NMR (DMSO-d6): δH 2.19, (6H, s, 2CH3), 2.46 (6H, s, 2CH3), 2.75–2.92 (4H, m, 2COCH2), 3.89–4.16 (4H, m, 2NCH2), 4.37–4.49 (2H, m, 2CH), 6.22 (2H, s, 2C=CH), 6.88–8.05 (12H, m, HAr). 13C-NMR (DMSO-d6) δC 13.2, 13.7 (CH3), 34.8 (CH), 34.9 (CH2CO), 49.8 (CH2N), 107.2, 111.3, 111.5, 113.9, 125.8, 129.1, 130.5, 134.1, 139.7, 143.6, 145.3, 151.8, 156.3, (Cpyrrazole, CAr), 171.4, 172.2, 179.7 (C=O). MS, m/z, (%) = 775 ([M+Na]+ 100); Anal. Calcd for C42H36N6O8 (752.79): C, 67.01; H, 4.82; N, 11.16. Found: C, 67.19; H, 4.91; N, 11.20.
N-(2,5-Dimethyl-1H-pyrrol-1-yl)-1-[3-({3-[3-(4-{[(2,5-dimethyl-1H-pyrrol-1-yl)amino]carbonyl}-2-oxo-1-pyrrolidinyl)phenoxy]-1,4-dioxo-1,4-dihydro-2-naphthalenyl}oxy)phenyl]-5-oxo-3-pyrrolid-inecarboxamide (11e). The crude product was chromatographed over a silica gel 60 column (2-propanone-hexane, 1:1), Rf 0.33. Orange crystals, (0.6 g, 23%), m.p. 193–194 °C; νmax/cm−1 1649, 1676, 1693, 1715 (C=O) and 3252 (NH). 1H-NMR (DMSO-d6): δH 1.97 (6H, s, 2CH3), 1.98 (6H, s, 2CH3), 2.65–2.93 (4H, m, 2COCH2), 3.37–3.49 (2H, m, 2CH), 3.87–4.11 (4H, m, 2NCH2), 5.64 (4H, s, 4CH), 6.90–8.07 (12H, m, HAr), 10.90 (2H, s, 2NH). 13C-NMR (DMSO-d6) δC 10.6 (4CH3), 33.6 (2CH), 35.5 (2CH2CO), 49.9 (2CH2N), 102.7, 107.1, 111.4, 113.7, 125.8, 126.4, 129.2, 130.5, 134.1, 139.8, 145.4, 156.4 (Cpyrrole, CAr), 171.4, 179.6 (C=O). MS, m/z, (%) = 803 ([M+Na]+ 100); Anal. Calcd for C44H40N6O8 (780.84): C, 67.68; H, 5.16; N, 10.76. Found: C, 67.59; H, 5.21; N, 10.79.
2-{3-[4-(1,2,3-Oxadiazol-5-yl)-2-oxo-1-pyrrolidinyl]phenoxy}-3-{3-[4-(1,3,4-oxadiazol-2-yl)-2-oxo-1-pyrrolidinyl]phenoxy}naphthoquinone (11f). The crude product was chromatographed over a silica gel 60 column (2-propanone-hexane, 5:1), Rf 0.23. Orange crystals, (0.52 g, 49%), m.p. 150–151 °C; νmax/cm−1 1578, 1604 (C=N), 1677 and 1705 (C=O). 1H-NMR (DMSO-d6): δH 2.82–3.08 (4H, m, 2COCH2), 3.99–4.26 (6H, m, CH, NCH2), 6.90–8.07 (12H, m, HAr), 9.21 (2H, s, 2N=CH). 13C-NMR (DMSO-d6) δC 27.4 (2CH), 35.9 (2CH2CO), 50.5 (2CH2N), 107.4, 111.9, 114.2, 126.0, 129.4, 130.8, 134.3, 139.9, 145.6, 154.8, 156.6, 166.2 (CAr, Coxadiazole), 171.2, 179.9 (C=O). MS, m/z, (%) = 667 ([M+Na]+ 100); Anal. Calcd for C34H24N6O8 (644.41): C, 63.35; H, 3.75; N, 13.04. Found: C, 63.22; H, 3.80; N, 12.97.
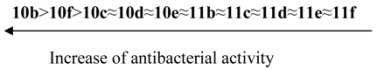
3.5. Antimicrobial Activity
The synthesized compounds were tested for their in vitro antimicrobial and antifungal activity against bacteria Escherichia coli B-906, Staphylococcus aureus 209-P, Mycobacterium luteum B-917 and fungi Candida tenuis VCM Y-70, Aspergillus niger VCM F-1119 by the diffusion method in agar (method A) and by the serial dilution method (method B).
Method А. Determination of antimicrobial and antifungal activity by diffusion method in agar. Antimicrobial and antifungal activity has been tested by diffusion in agar on solid nutrient medium (beef-extract agar for bacteria, wort agar for fungi). Petri plates containing 20 mL of nutrient medium were used for all tested microorganisms. The inoculums (the microbial loading − 10
9 cells (spores)/1 mL) was spread on the surface of the solidified media and Whatman no.1 filter paper discs (6 mm in diameter) impregnated with the test compound solution (0.1% and 0.5%) were placed on the plates. The duration of bacteria incubation was 24 h at 35 °C and the one of fungi incubation was 48–72 h at 28–30 °C. The antimicrobial effect and degree of activity of the tested compounds were evaluated by measuring the zone diameters. The results were compared with well known drugs (
Table 1). Every experiment was repeated three times.
Method B. Determination of minimal inhibitory (MIC), minimal bactericidal (MBC) and minimal fungicidal (MFC) concentrations using serial dilution method. The tested compounds were added to the nutrient medium (beef-extract broth for bacteria and wort for fungi) as solutions in dimethyl sulfoxide (DMSO) by ensuring needed concentration (0.9–500.0 μg/mL). Bacteria and fungi inoculum was inoculated into nutrient medium (the microbial loading was 106 cells (spores)/1 mL). The duration of bacteria incubation was 24 h at 35 °C and the one of fungi incubation was 48–72 h at 28–30 °C. The results were estimated by the microorganism growth measured by degree of microbial turbidity in nutrient medium. Minimal inhibitory concentration (MIC) of any compound is defined as the lowest concentration which completely inhibits visible growth (turbidity on liquid nutrient medium).
Determination of MBC and MFC. Visually transparent nutrient medium solutions were sowed on the sterile agar medium (beef-extract agar for bacteria, wort agar for fungi). The duration of bacteria incubation was 24 h at 35 °C and the one of fungi incubation was 48–72 h at 28–30 °C. In the absence of microorganism colony growth on the incubated Petri plate, minimal bactericidal (MBC) and minimal fungicidal (MFC) concentrations of the investigated compounds were identified. The test was repeated three times.