Ionic Liquid-Based Microwave-Assisted Extraction of Flavonoids from Bauhinia championii (Benth.) Benth.
Abstract
:Abbreviations
| IL | ionic liquid |
| MAE | microwave-assisted extraction |
| CHRE | conventional heat-reflux extraction |
1. Introduction
2. Results and Discussion
2.1. Compositions of Bauhinia championii (Benth.) Benth
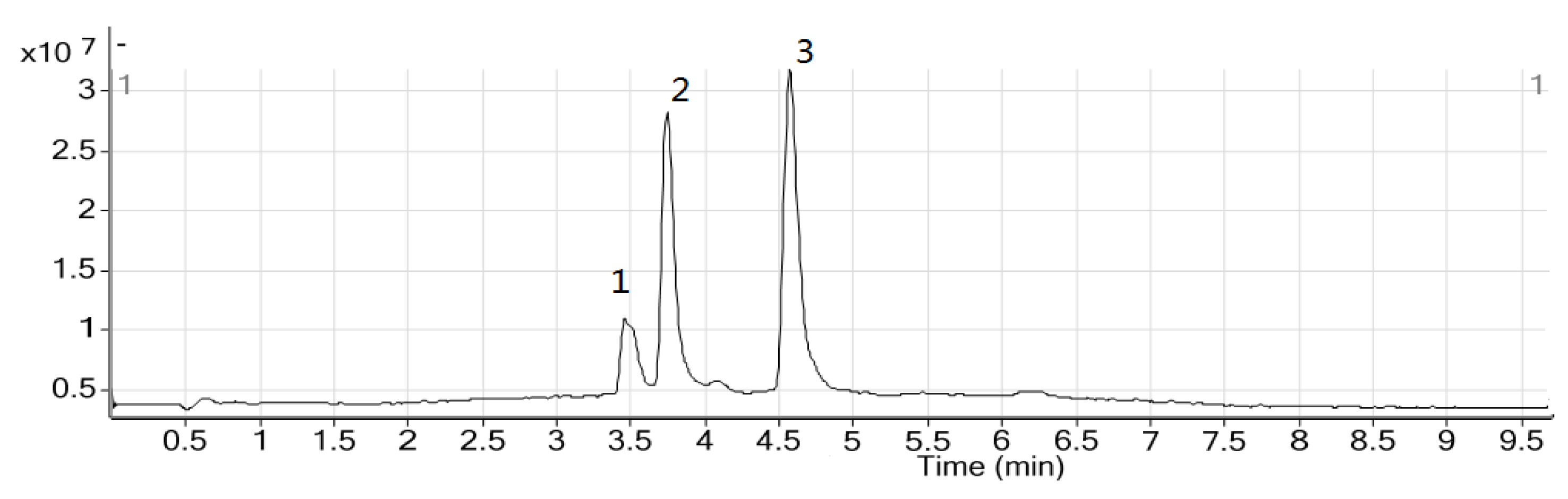
2.2. HPLC Analysis of Sample and Quantification

2.3. Screening of ILs
| Solvent | Yield (mean ± SD, mg/g) | ||
|---|---|---|---|
| Myricetin | Quercetin | Kaempferol | |
| [bmim] Br | 0.0497 ± 0.02 | 0.2375 ± 0.02 | 0.0305 ± 0.03 |
| [bmim] Cl | 0.0103 ± 0.01 | 0.1257 ± 0.04 | 0.0211 ± 0.01 |
| [bmim] [PF6] | 0.0163 ± 0.02 | 0.1078 ± 0.01 | 0.0718 ± 0.01 |
| [bmim] [BF4] | 0.0411 ± 0.03 | 0.1065 ± 0.01 | 0.0190 ± 0.02 |
| [bmim] [H2PO4] | 0.0194 ± 0.03 | 0.1035 ± 0.03 | 0.0289 ± 0.02 |
| [bmim]2 [SO4] | 0.0110 ± 0.01 | 0.0953 ± 0.05 | 0.0114 ± 0.04 |
| [bmim] [HSO4] | 0.0200 ± 0.01 | 0.1453 ± 0.05 | 0.0274 ± 0.04 |
| [hmim] Br | 0.0127 ± 0.01 | 0.1329 ± 0.03 | 0.0181 ± 0.03 |
| [emim] Br | 0.0211 ± 0.03 | 0.1556 ± 0.01 | 0.0390 ± 0.02 |
| [HOOCCH2-mim] Cl | 0.0174 ± 0.01 | 0.1375 ± 0.02 | 0.0225 ± 0.01 |
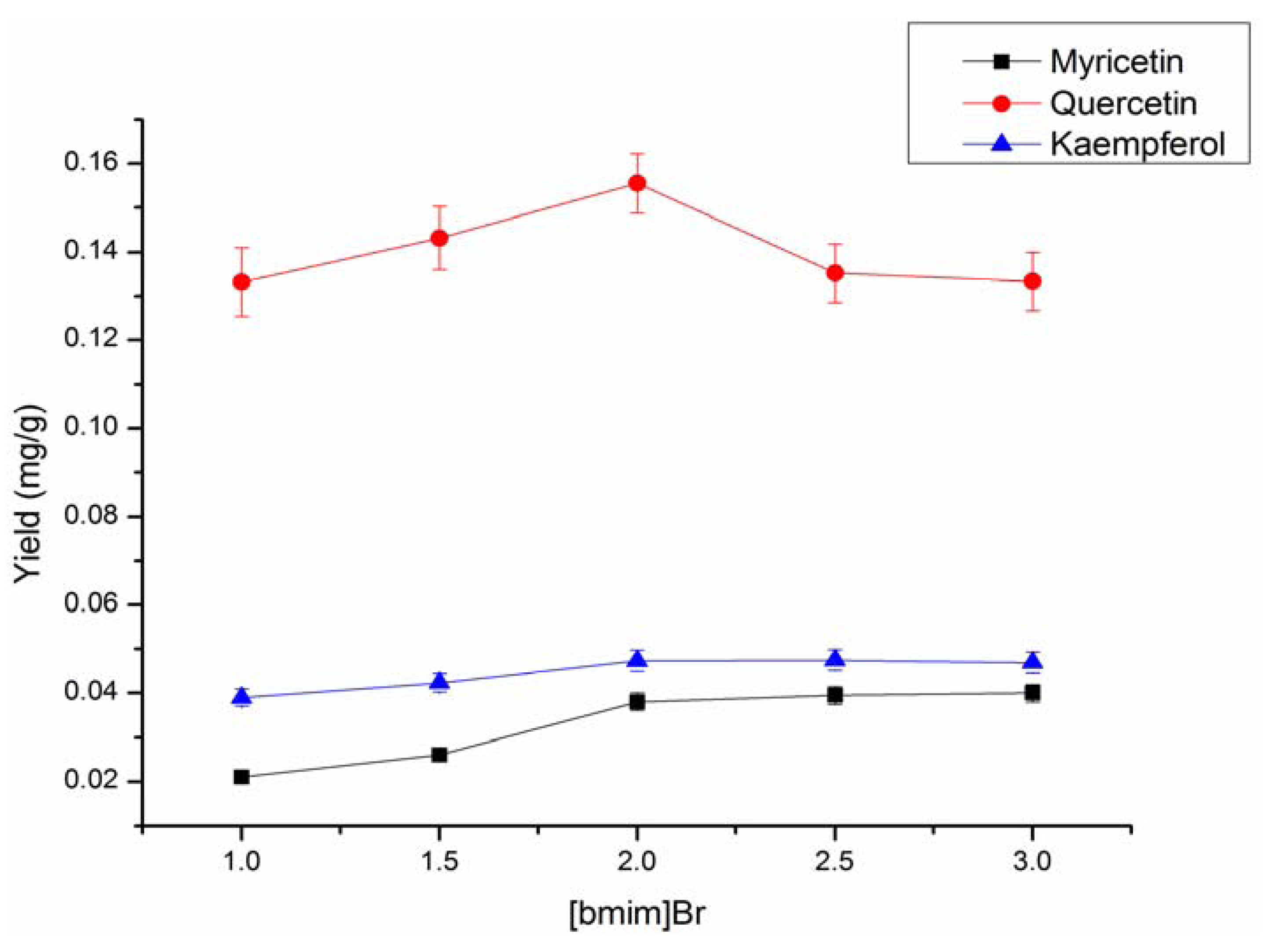
2.4. Optimization of ILs-MAE Extraction Conditions

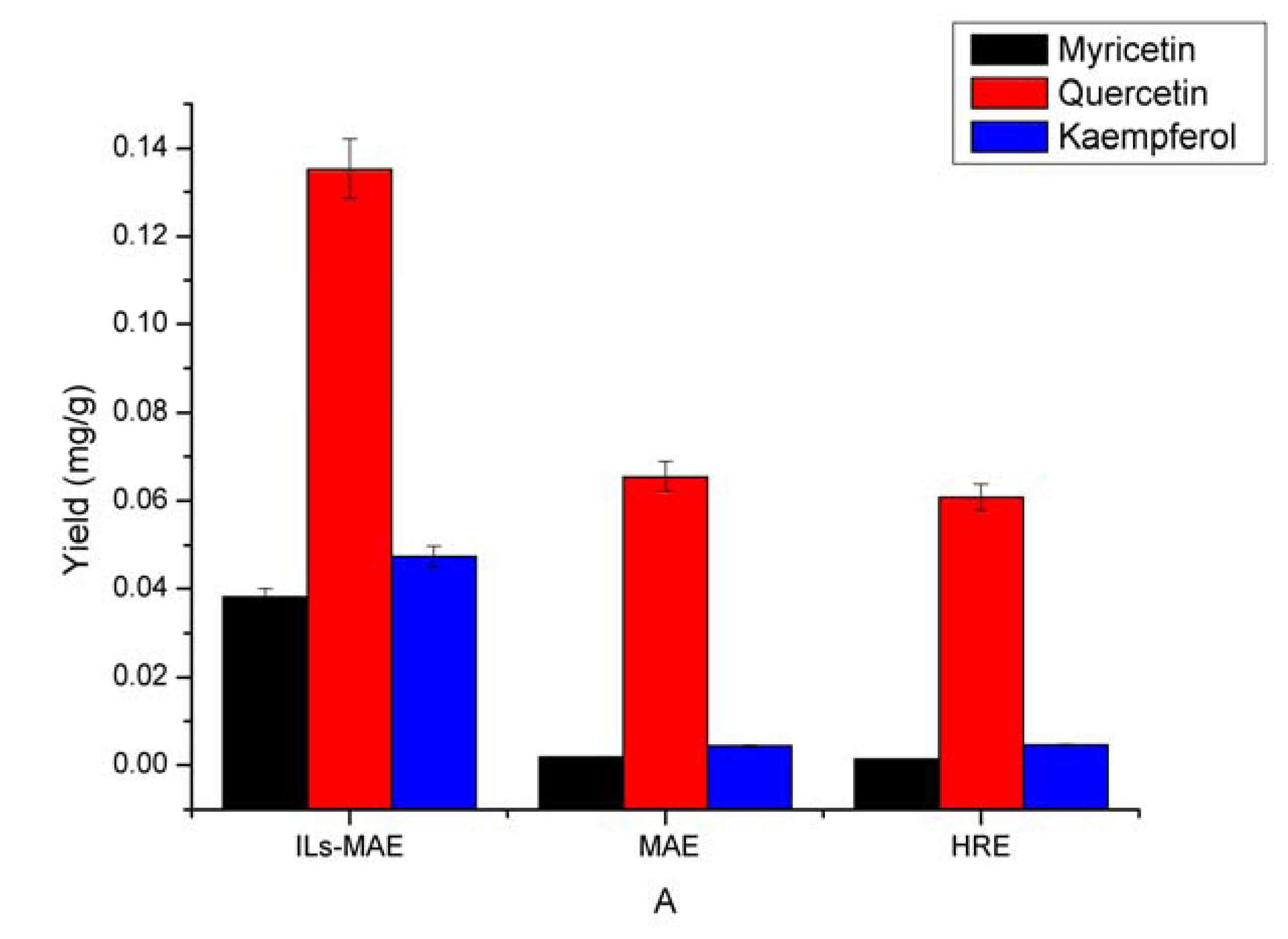
2.5. Comparison of the Proposed ILs-MAE Approach with the Other Conventional Extraction Methods
2.6. Method Validation
| Analytes | Linearities | Correlation coefficients | Calibration range (μg·mL−1) |
|---|---|---|---|
| Myricetin | Y = 0.583x − 0.5086 | R2 = 0.9996 | X = 30–110 |
| Quercetin | Y = 0.7856x − 21.681 | R2 = 0.9998 | X = 80–300 |
| Kaempferol | Y = 0.1789x + 53.997 | R2 = 0.9991 | X = 10–110 |
3. Experimental
3.1. Reagents and Materials
3.2. Flavonoid Extractions from Bauhinia Championii (Benth.) Benth
3.2.1. Ionic Liquid-Based Microwave-Assisted Extraction (IL-MAE)
3.2.2. Conventional Reference Extraction Method
3.2.3. Microwave-Assisted Extraction (MAE)
3.3. HPLC Analysis
3.4. LC-MS-MS Identification
| t/min | A | B |
|---|---|---|
| 0 | 30 | 70 |
| 1.8 | 10 | 90 |
| 2.5 | 10 | 90 |
| 5.01 | 30 | 70 |
3.5. Statistical Methods
4. Conclusions
Acknowledgements
References
- Jiangsu New Medical College, Dictionary of Chinese Traditional Drug; Shanghai Scientific and Technical Publishers: Shanghai, China, 1986; p. 43.
- Fang, D.; Luo, J.Y.; Su, G.X.; Tao, Y.P.; Tan, X.Y.; Tan, D.H. Selectional Medications Prescription of Zhuang Folk Medicine; Guangxi Nationalities Publishing House: Guangxi, China, 1985; pp. 6–7. [Google Scholar]
- Wei, H.S.; Qi, Z.Y. Curative Effect Observation of Fumigation treatment Arthralgia 58 Cases. J. Ther. TCM 1998, 7, 41–42. [Google Scholar]
- Xie, G.C.; Xiao, Q.Q. Chinese Illuminations of Well-Triedrecipe of Herbs; Shantou University Press: Shantou, China, 2000; p. 125. [Google Scholar]
- Guo, C.Q.; Xiang, R.M. Treatment of acute or chronic backleg pain by yangtiteng. Chin. Folk Med. 2001, 9, 61. [Google Scholar]
- Hu, J.; Ye, H.Z.; Hun, Y.; Zhou, C.H. Effects of three Fujian native herbs on platelet aggregation of sd rat in vitro. J. Fujian College TCM 2007, 17, 23–26. [Google Scholar]
- Hong, Z.F.; Zheng, H.Y.; Xu, W.; Wang, R.G. Research of anti-inflammatory and analgesic effects of Kangmeifu MEBO. Chin. J. Tradit. Med. Sci. Technol. 2007, 14, 410–411. [Google Scholar]
- Hong, Z.F.; Zheng, H.Y.; Xu, W.; Wang, R.G. Antimicrobial activity of Kangmeifu MEBO. Chin. J. Microecol. 2007, 19, 177–178. [Google Scholar]
- Bai, Y.H.; Zhan, Q.F.; Xia, Z.H.; Lao, A.N. Studies on the chemical constituents of Bauhinia championii (Benth.) (II). Nat. Prod. Res. Devel. 2004, 312–313. [Google Scholar]
- Bai, Y.H.; Zhan, Q.F.; Lao, A.N.; Xia, Z.H. Studies on the chemical constituents of Bauhinia championii (Benth.)(I). Chin. J. Chin. Mater. Med. 2005, 30, 42–43. [Google Scholar]
- Ye, H.Z; Zheng, C.S; Lin, W. Component Analysis of Volatile Oil From Bauhinia championii (Benth.) Benth. by GC-MS. J. Fujian College TCM 2009, 19, 20–22. [Google Scholar]
- Ye, H.Z; Yang, M.P; Lin, W. Determination of Polysaccharide in Bauhinia championii (Benth.) Benth. J. Fujian College TCM 2009, 19, 22–24. [Google Scholar]
- He, Z.; Xia, W. Microwave-assisted extraction of phenolics from Canarium album L. and identification of the main phenolic compound. Nat. Prod. Res. 2011, 25, 85–92. [Google Scholar] [CrossRef]
- Li, D.C.; Jiang, J.G. Optimization of the microwave-assisted extraction conditions of tea polyphenols from green tea. Int. J. Food Sci. Nutr. 2010, 61, 837–845. [Google Scholar] [CrossRef]
- Alipieva, K.; Petreska, J.; Gil-Izquierdo, A.; Stefova, M.; Evstatieva, L.; Bankova, V. Influence of the extraction method on the yield of flavonoids and phenolics from Sideritis spp. (Pirin Mountain tea). Nat. Prod. Commun. 2010, 5, 51–54. [Google Scholar]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2165. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar]
- Liu, J.F.; Jönsson, J.Å.; Jiang, G.B. Application of ionic liquids in analytical chemistry. Trends Anal. Chem. 2005, 24, 20–27. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Li, G.K. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati. J. Chromatogr. A 2007, 1140, 56–62. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Luo, X.J.; Li, G.K. Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. Talanta 2009, 78, 1177–1184. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Li, G.K. Microwave-assisted extraction of alkaloids in Lycoris Radiata using ionic liquids solution. Chin. J. Anal. Chem. 2007, 35, 1570–1574. [Google Scholar]
- Lu, Y.; Ma, W.; Hu, R.; Dai, X.; Pan, Y. Ionic liquid-based microwave-assisted extraction of phenolic alkaloids from the medicinal plant Nelumbo nucifera Gaertn. J. Chromatogr. A 2008, 1208, 42–46. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Rogers, R.D. Room Temperature ionic liquids as novel media for ‘clean’ liquid-liquid exuacfion. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Zhang, S.J.; Lv, X.M.; Kou, Y. Ionic Liquid from Basic Research and Industry; Applications. Science Press: Beijing, China, 2006; pp. 183–192. [Google Scholar]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing ionic liquids on the basis of multiple solvation interactions. J. Amer. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar]
- Guo, Z.; Lue, B.M.; Thomasen, K.; Meyer, A.S.; Xu, X.B. Predictions of flavonoid solubility in ionic liquids by COSMO-RS: Experimental verification, structural elucidation, and solvation characterization. Green Chem. 2007, 9, 1362–1373. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Li, G.K. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati. J. Chromatogr. A 1140, 56–62. [Google Scholar]
- Li, C.Z.; Wang, Q.; Zhao, Z.B.K. Acid in ionic liquid: An efficient system for hydrolysis of lignocellulose. Green Chem. 2008, 10, 177–182. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Li, G.K. Ionic liquid aqueous solvent-based microwave-assisted hydrolysis for the extraction and HPLC determination of myricetin and quercetin from Myrica rubra leaves. Biomed. Chromatogr. 2011, 25, 472–478. [Google Scholar]
- Wang, J.X.; Xiao, X.H.; Li, G.K. Study of vacuum microwave-assisted extraction of polyphenolic compounds and pigment from Chinese herbs. J. Chromatogr. A 2008, 1198–1199, 45–53. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, W.; Chu, K.; Li, H.; Zhang, Y.; Zheng, H.; Chen, R.; Chen, L. Ionic Liquid-Based Microwave-Assisted Extraction of Flavonoids from Bauhinia championii (Benth.) Benth. Molecules 2012, 17, 14323-14335. https://doi.org/10.3390/molecules171214323
Xu W, Chu K, Li H, Zhang Y, Zheng H, Chen R, Chen L. Ionic Liquid-Based Microwave-Assisted Extraction of Flavonoids from Bauhinia championii (Benth.) Benth. Molecules. 2012; 17(12):14323-14335. https://doi.org/10.3390/molecules171214323
Chicago/Turabian StyleXu, Wei, Kedan Chu, Huang Li, Yuqin Zhang, Haiyin Zheng, Ruilan Chen, and Lidian Chen. 2012. "Ionic Liquid-Based Microwave-Assisted Extraction of Flavonoids from Bauhinia championii (Benth.) Benth." Molecules 17, no. 12: 14323-14335. https://doi.org/10.3390/molecules171214323




