Isolation, Identification and Antimicrobial Activities of Two Secondary Metabolites of Talaromyces verruculosus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Identification
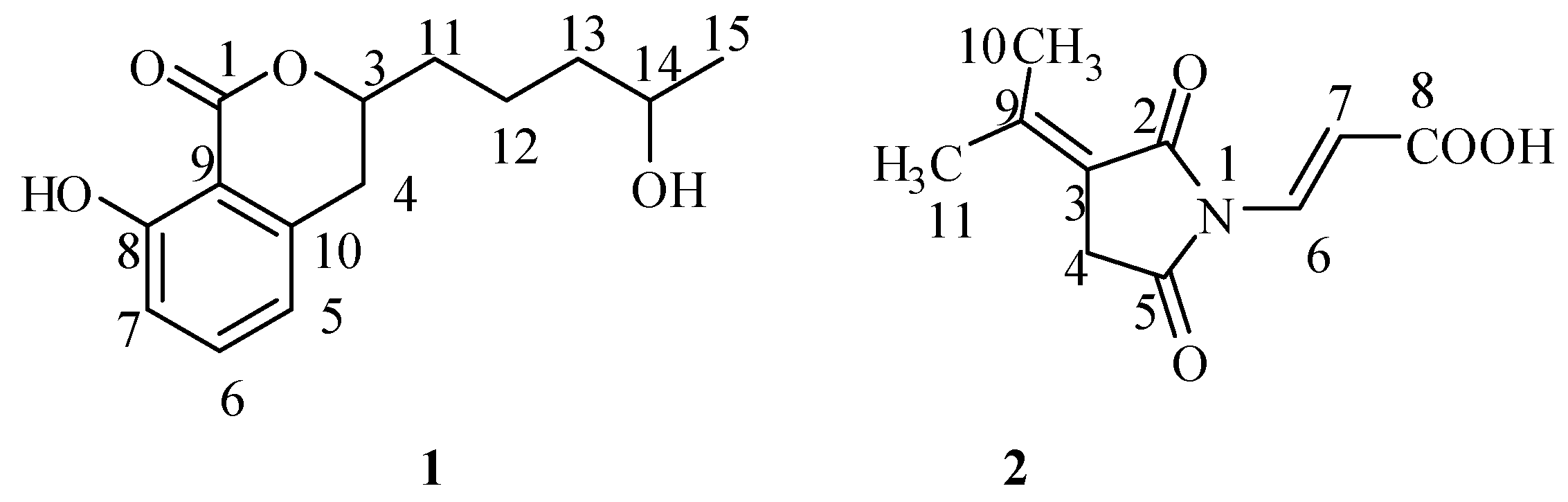
2.2. Bioactivity
| Compound | Growth inhibition (%, mean ± SD) | MIC (µg mL−1) | ||||
|---|---|---|---|---|---|---|
| A. solani | V. mali | C. lunata | B. berengeriana | S. aureus | E. coli | |
| 1 | 92.6 ± 2.1 | 97.3 ± 3.3 | 87.2 ± 2.8 | 94.9 ± 3.9 | 2.5 | 5.0 |
| 2 | N a (16.3 ± 5.4) b | N (21.3 ± 4.6) | N (25.6 ± 5.4) | N (19.7 ± 3.8) | >100 | >100 |
| Thiabendazole | 54.7 ± 2.6 | 100 ± 0.0 | 51.5 ± 4.1 | 92.8 ± 3.7 | ― | ― |
| Ceftriaxone sodium | ― | ― | ― | ― | 0.625 | 0.625 |
3. Experimental
3.1. General
3.2. Plant and Fungal Strain Materials
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antibacterial Assay
3.6. Antifungal Assay
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
References
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Trejo-Estrada, S.R.; Paszczynski, A.; Crawford, D.L. Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9. J. Ind. Microbiol. Biotech. 1998, 21, 81–90. [Google Scholar] [CrossRef]
- Smalla, K.; Wachtendorf, U.; Heuer, H.; Liu, W.-T.; Forney, L. Analysis of BIOLOGGN substrate utilization patterns by microbial communities. Appl. Environ. Microbiol. 1998, 64, 1220–1225. [Google Scholar]
- Grayston, S.J.; Wang, S.Q.; Campbell, C.D.; Edwards, A.C. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 1998, 30, 369–378. [Google Scholar] [CrossRef]
- Rovira, A.D. Plant root excretions in relation to the rhizosphere effect I. Plant Soil 1956, 7, 178–194. [Google Scholar] [CrossRef]
- Berg, G.; Zachow, C.; Lottmann, J.; Gotz, M.; Costa, R.; Smalla, K. Impact of plant species and site on rhizosphere-associated fungi antagonistic to Verticillium dahliae Kleb. Appl. Environ. Microbiol. 2005, 71, 4203–4213. [Google Scholar] [CrossRef]
- Rovira, A.D. Root excretions in relation to the rhizosphere effect. IV. Influence of plant species, age of plant, light, temperature, and calcium nutrition on exudation. Plant Soil 1959, 11, 53–64. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- He, J.; Wijeratne, E.M.; Bashyal, B.P.; Zhan, J.; Seliga, C.J.; Liu, M.X.; Pierson, E.E.; Pierson, L.S., III; VanEtten, H.D.; Gunatilaka, A.A. Cytotoxic and other metabolites of Aspergillus inhabiting the rhizosphere of Sonoran desert plants. J. Nat. Prod. 2004, 67, 1985–1991. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hino, T.; Yata, S.; Itoh, T.J.; Sato, H.; Kawazu, K. Unique spindle poisons, curvularin and its derivatives, isolated from Penicillium specie. Agric. Biol. Chem. 1988, 52, 3119–3123. [Google Scholar] [CrossRef]
- Zhou, G.X.; Wijeratne, E.M.; Bigelow, D.; Pierson, L.S., III; VanEtten, H.D.; Gunatilaka, A.A. Aspochalasins I, J, and K: Three new cytotoxic cytochalasans of Aspergillus flavipes from the rhizosphere of Ericameria laricifolia of the Sonoran Desert. J. Nat. Prod. 2004, 67, 328–332. [Google Scholar] [CrossRef]
- Wijeratne, E.M.; Turbyville, T.J.; Zhang, Z.; Bigelow, D.; Pierson, L.S., III; VanEtten, H.D.; Whitesell, L.; Canfield, L.M.; Gunatilaka, A.A. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the sonoran desert. J. Nat. Prod. 2003, 66, 1567–1573. [Google Scholar] [CrossRef]
- Turbyville, T.J.; Wijeratne, E.M.; Whitesell, L.; Gunatilaka, A.A. The anticancer activity of the fungal metabolite terrecyclic acid A is associated with modulation of multiple cellular stress response pathways. Mol. Cancer Ther. 2005, 4, 1569–1576. [Google Scholar] [CrossRef]
- Wijeratne, E.M.; Carbonezi, C.A.; Takahashi, J.A.; Seliga, C.J.; Turbyville, T.J.; Pierson, E.E.; Pierson, L.S., III; VanEtten, H.D.; Whitesell, L.; Bolzani, Vda S.; et al. Isolation, optimization of production and structure-activity relationship studies of monocillin I, the cytotoxic constituent of Paraphaeosphaeria quadriseptata. J. Antibiot. 2004, 57, 541–546. [Google Scholar] [CrossRef]
- Zhan, J.; Wijeratne, E.M.; Seliga, C.J.; Zhang, J.; Pierson, E.E.; Pierson, L.S., III; VanEtten, H.D.; Gunatilaka, A.A. A new anthraquinone and cytotoxic curvularins of a Penicillium sp. from the rhizosphere of Fallugia paradoxa of the Sonoran desert. J. Antibiot. 2004, 57, 341–344. [Google Scholar] [CrossRef]
- Samson, R.A.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvad, J.C. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef]
- Yang, L.Z.; Zhou, L.; Xu, H.; Qin, B.F. Isolation and identification and antifungal activity of a Penicillium. Acta Agric. Boreali-Occid. Sin. 2009, 18, 98–102. [Google Scholar]
- Gao, G.C.; Qi, S.H.; Zhang, S.; Yin, H.; Xiao, Z.H.; Li, M.Y.; Li, Q.X. Phenolic compounds and iridoids from the stem bark of Chinese mangrove associate Catunaregam spinosa. Pharmazie 2008, 63, 542–544. [Google Scholar]
- Miao, F.; Qin, B.F.; Yang, L.Z.; Yang, X.J.; Zhou, L. (E)-3-[2,5-Dioxo-3-(propan-2-ylidene) pyrrolidin-1-yl]acrylic acid. Acta Cryst. 2010, E66, o634. [Google Scholar]
- Guz, N.R.; Stermitz, F.R.; Johnson, J.B.; Beeson, T.D.; Willen, S.; Hsiang, J.-F; Lewis, K. Flavonolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: structure-activity relationships. J. Med. Chem. 2001, 44, 261–268. [Google Scholar] [CrossRef]
- Zhang, J.W.; Li, S.K.; Wu, W.J. The main chemical composition and in vitro antifungal activity of the essential oils of ocimum basilicum Linn. var. pilosum (Willd.) Benth. Molecules 2009, 14, 273–278. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miao, F.; Yang, R.; Chen, D.-D.; Wang, Y.; Qin, B.-F.; Yang, X.-J.; Zhou, L. Isolation, Identification and Antimicrobial Activities of Two Secondary Metabolites of Talaromyces verruculosus. Molecules 2012, 17, 14091-14098. https://doi.org/10.3390/molecules171214091
Miao F, Yang R, Chen D-D, Wang Y, Qin B-F, Yang X-J, Zhou L. Isolation, Identification and Antimicrobial Activities of Two Secondary Metabolites of Talaromyces verruculosus. Molecules. 2012; 17(12):14091-14098. https://doi.org/10.3390/molecules171214091
Chicago/Turabian StyleMiao, Fang, Rui Yang, Dong-Dong Chen, Ying Wang, Bao-Fu Qin, Xin-Juan Yang, and Le Zhou. 2012. "Isolation, Identification and Antimicrobial Activities of Two Secondary Metabolites of Talaromyces verruculosus" Molecules 17, no. 12: 14091-14098. https://doi.org/10.3390/molecules171214091




