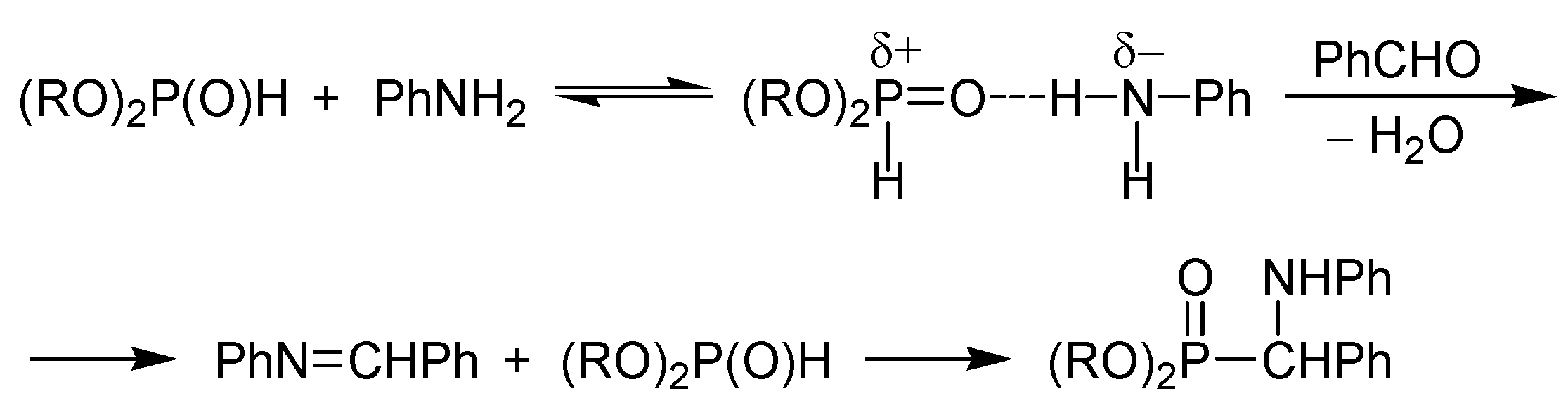The Kabachnik–Fields Reaction: Mechanism and Synthetic Use
Abstract
:1. Introduction

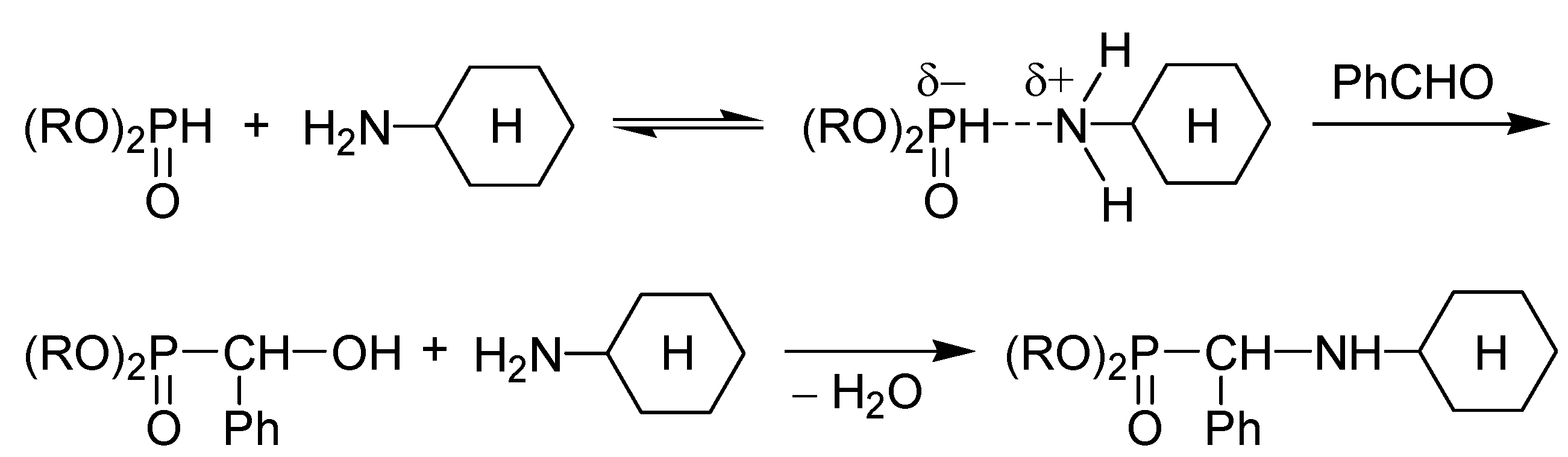
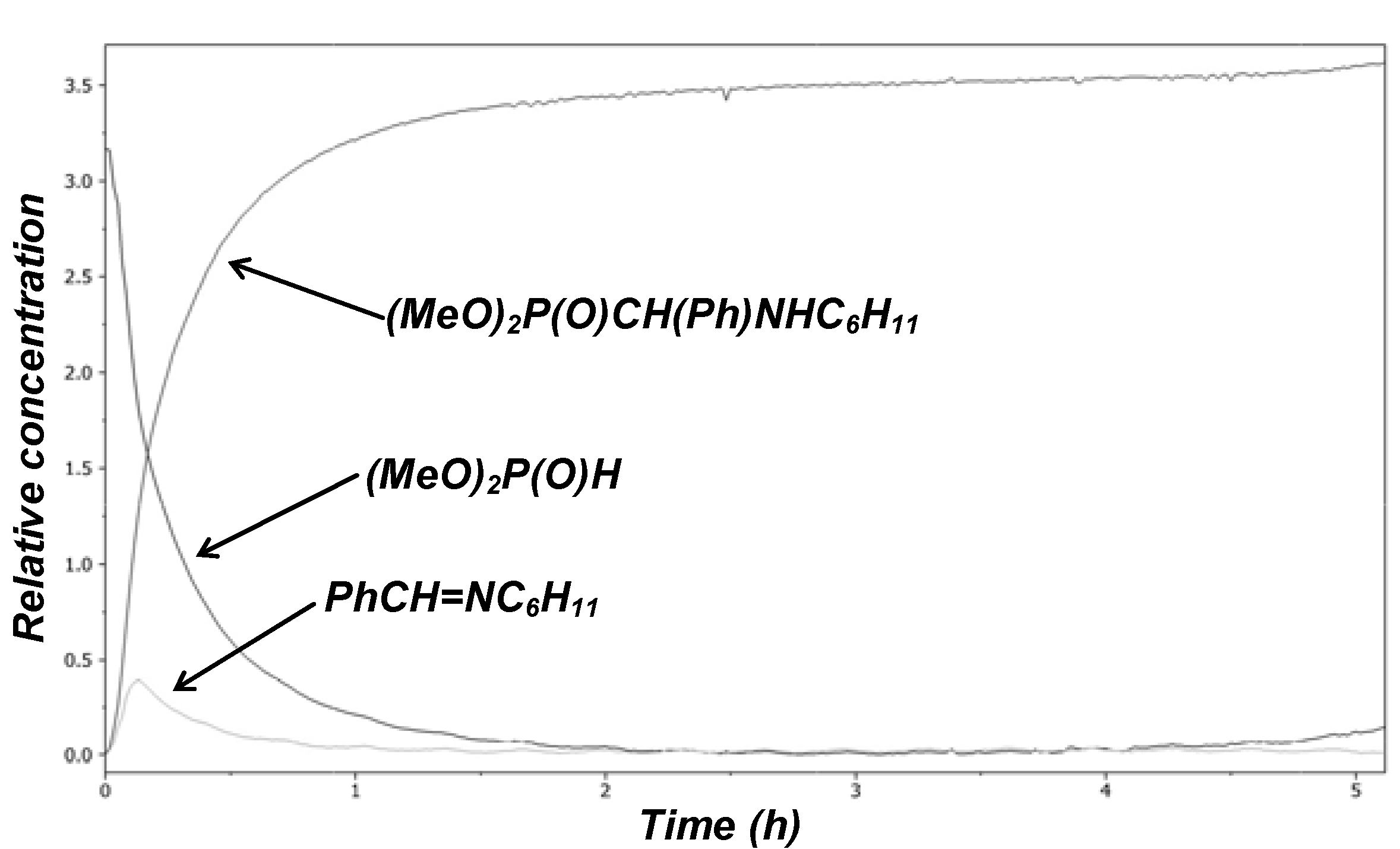
2. Possible Pathways for the Kabachnik–Fields Reaction




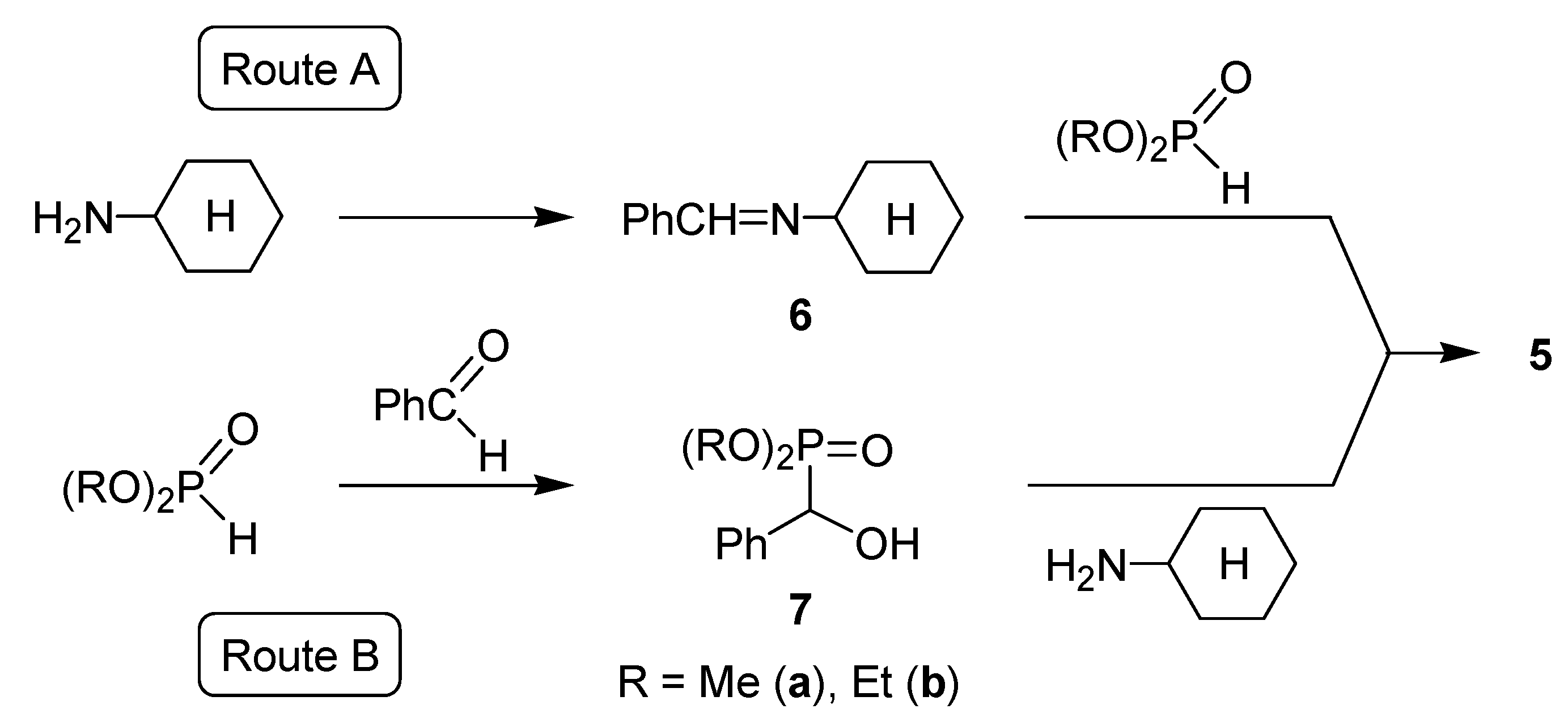
| Species | Relative energy (kJ/mol) |
|---|---|
| Reactants (benzaldehyde, cyclohexylamine and dimethyl phosphite) | 0.0 |
| Imine intermediate 6 | –18.6 |
| α-Hydroxyphosphonate intermediate 7 | –40.5 |
| Product 5 | –42.9 |
3. Microwave-Assisted Solvent- and Catalyst-Free Approach for the Synthesis of α-Amino-phosphonates and Related Derivatives
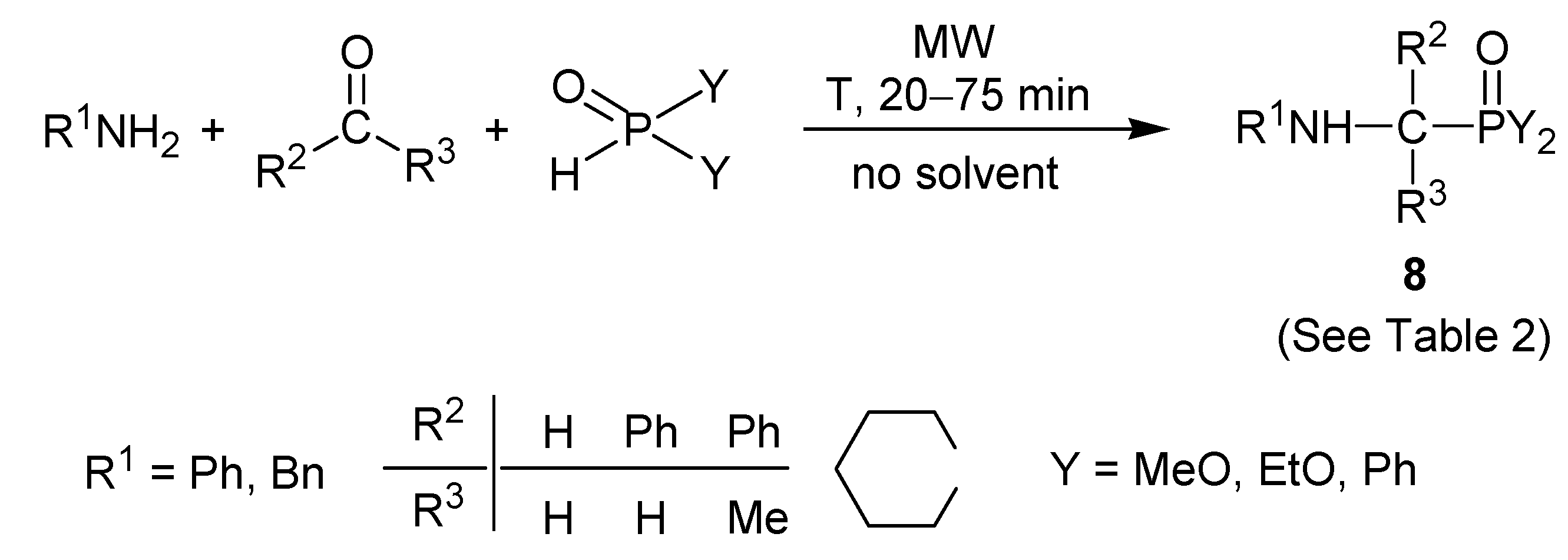
| Entry | R1 | R2 | R3 | Y | Product | T (°C) | Yield (%) | Yield (%) of catalytic methods [ref.] † |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | H | H | EtO | 8a | 80 a | 91 | |
| 100 b | ||||||||
| 2 | Ph | H | H | MeO | 8b | 80 a | 80 | |
| 80 b | ||||||||
| 3 | Ph | H | H | Ph | 8c | 80 | 94 | |
| 4 | Bn | H | H | EtO | 8d | 100 | 81 | |
| 5 | Bn | H | H | Ph | 8e | 80 | 88 | |
| 6 | Ph | H | Ph | EtO | 8f | 100 | 93 | 98 [27], 85 [28], ~95 [29], 88 [30], 79 [31], 93 [32], 92 [33], ~90 [34], 96 [35], 60 [36], 86 [37], 92 [38] |
| 7 | Ph | H | Ph | MeO | 8g | 100 | 86 | 98 [24], 98 [27], 92 [33] |
| 8 | Ph | H | Ph | Ph | 8h | 80 | 87 | |
| 9 | Bn | H | Ph | EtO | 8i | 100 | 83 | 99 [28], 84 [29], 92 [30], 85 [31], 91 [32], 91 [33], 92 [35], 92 [36], 93 [38] |
| 10 | Bn | H | Ph | MeO | 8j | 100 | 87 | 95 [27], 82 [33] |
| 11 | Ph | Me | Ph | EtO | 8k | 120 | 80 | 75 [27], 74 [30], 63 [35], 18 [36] |
| 12 | Bn | Me | Ph | EtO | 8l | 120 | 84 | 92 [26], 80 [27], 81 [38] |
| 13 | Bn | Me | Ph | Ph | 8m | 100 a | 80 | |
| 120 b | 80 | |||||||
| 14 | Ph |  | EtO | 8n | 120 | 81 | 92 [27], ~72 [29], 86 [30], 47 [31], 87 [37] | |
| 15 | Bn |  | EtO | 8o | 120 | 91 | 85 [26], 90 [27], 83 [31], 80 [33], 85 [38] | |
| 16 | Bn |  | MeO | 8p | 120 | 85 | 92 [27] | |
| 17 | Bn |  | Ph | 8q | 100 a | 80 | ||
| 120 b |
| Catalyst | Solvent | MW/Δ | T [°C] | t | Yield (Product) [%] | Ref. |
|---|---|---|---|---|---|---|
| Phthalocyanine-AlCl | CH2Cl2 | – | 26 a | 12 h | 92 (8b), 85 (8p) | [26] |
| Mg(ClO4)2 | – | – | 26 | 2 min/8 h | 90–98 ( 8f, 8g, 8j, 8n-p) | [27] |
| Mg(ClO4)2 | – | Δ | 50-80 | 45 min–12 h | 80–99 (8f, 8i, 8l,8n-p) | [27,28] |
| Mg(ClO4)2 | EtOH | Δ | 50 | 5 h/12 h | 85 (8f), 99 (8i) | [28] |
| M(OTf)n M = Li, Mg, Al, Cu, Ce | – | Δ | 80 | 20 min–3.5 h | 72–95 (8f, 8i, 8n) | [29] |
| GaI3 | CH2Cl2 | – | 26 | 3–6 h | 74–92 (8f, 8i, 8k, 8n) | [30] |
| In(OTf)3 | THF | Δ | 66 | 21–35 h | 47–85 (8f, 8i, 8n, 8o) | [31] |
| BiNO3 | – | – b | 26 | 10 h | 93 (8f), 91 (8i) | [32] |
| BiCl3 | MeCN | Δ | 80 | 6–15 h | 80–92 (8f, 8g, 8i, 8j, 8o) | [33] |
| FeCl3 | EtOH (or solvent free) | – | 26 | ~90 (8f) | [34] | |
| YbCl3 | MeCN | – | 26 | 24 h | 63–96 (8f, 8i, 8k) | [35] |
| SmI2 (+ 4 Å mol sieves) | MeCN | Δ | 80 | 24 h | 18–92 (8f, 8i, 8k) | [36] |
| ceric ammonium nitrate | MeCN | – | 26 | 3 h | 86 (8f), 87 (8n) | [37] |
| InCl3 | THF | Δ | 66 | 9–12 h | 81–93 (8f, 8i, 8l, 8o) | [38] |
| InCl3 | DMF | MW | no data | 2 min | 82 (8f) c | [39] |
| InCl3 | [bmim][PF6] | MW | no data | 2 min | 91 (8f) c | [39] |
| Ln(OTf)3 Ln = Yb, Sc, Dy, Sm | DMF | MW | no data | 2 min | 72 ( 8f) c | [39] |
| Ln(OTf)3 Ln = Yb, Sc, Dy, Sm | [bmim][PF6] | – | 26 | 27 h | 92 (8f) c | [40] |
| Ln(OTf)3 Ln = Yb, Sc, Dy, Gd | [bmim][PF6] | MW | no data | 2 min | 89 (8f) c | [39] |
| the solvent acts as catalyst | [bmim][BF4] | – | 26 | 5 h/8 h | 90 (8f), 84 (8f) | [41] |
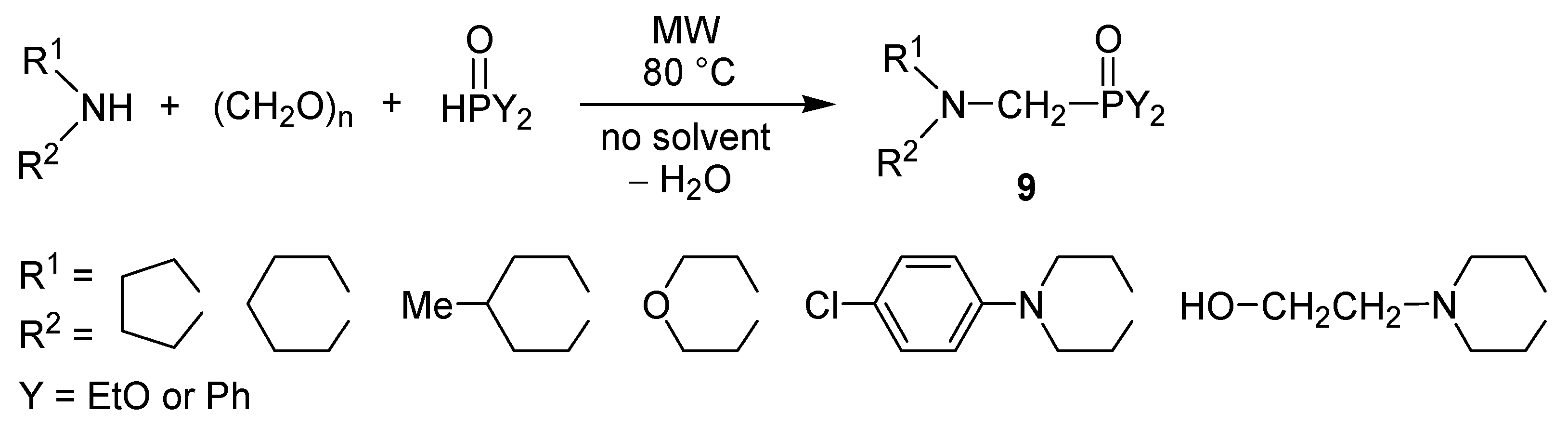
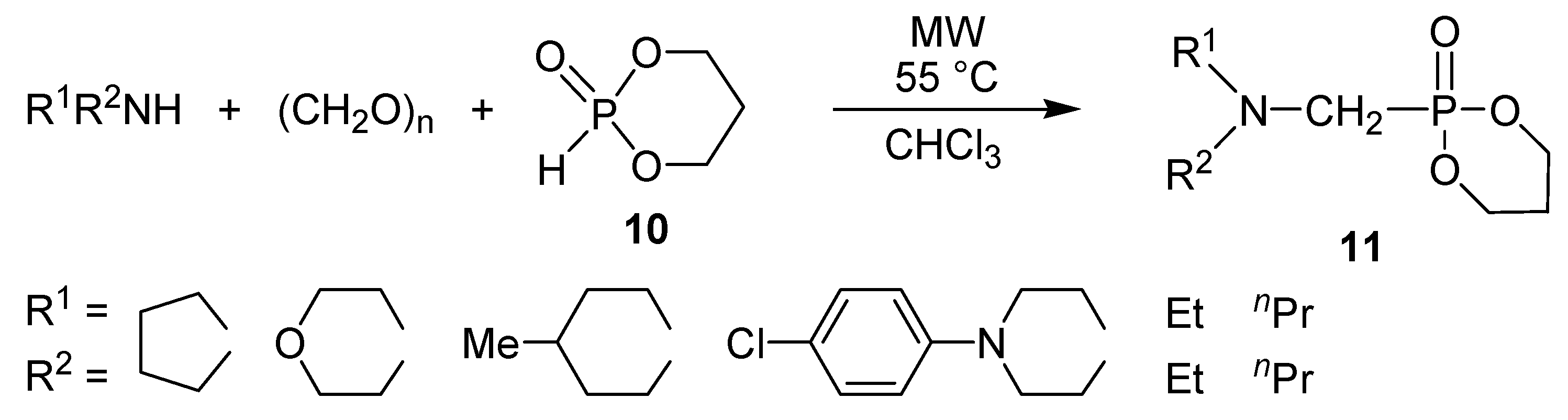
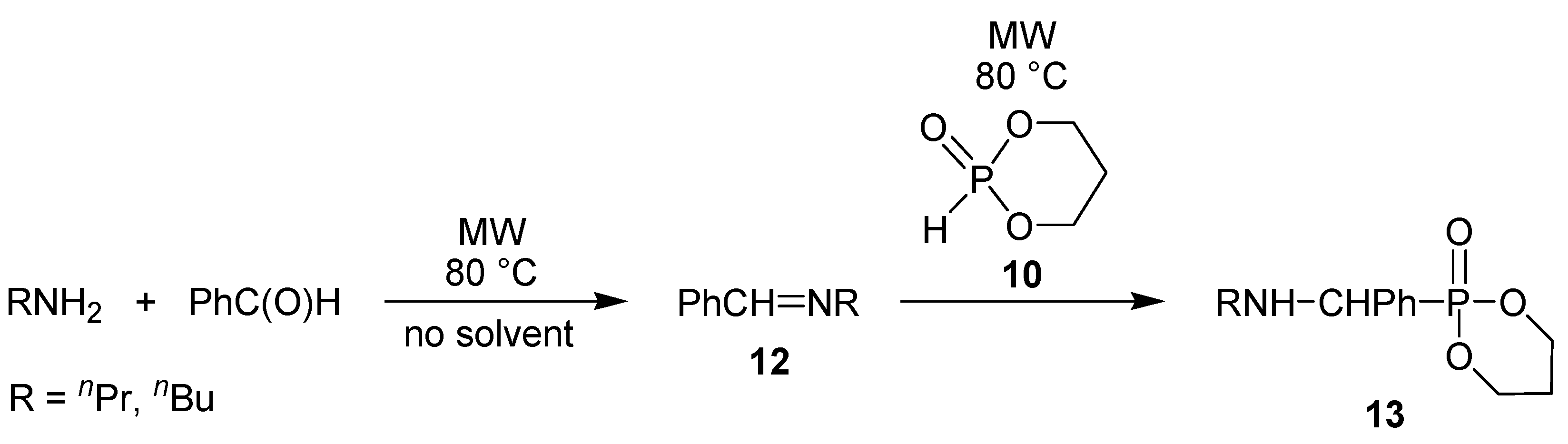
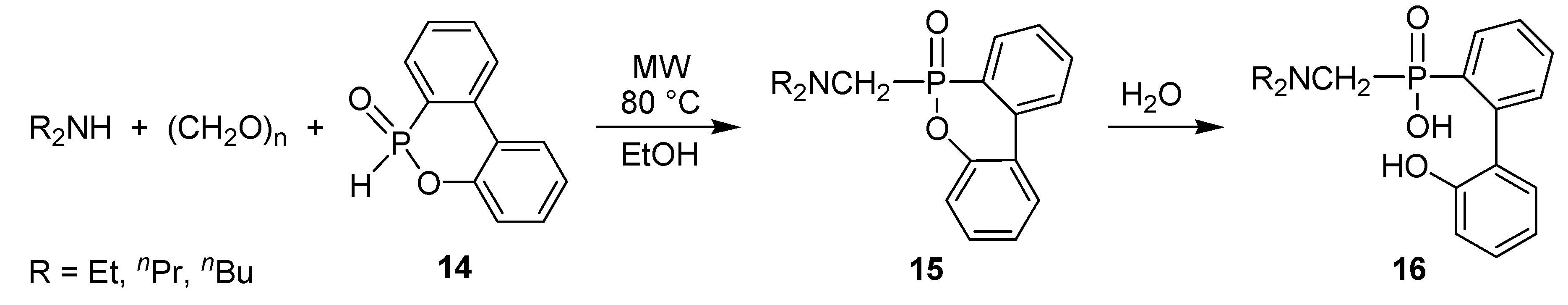


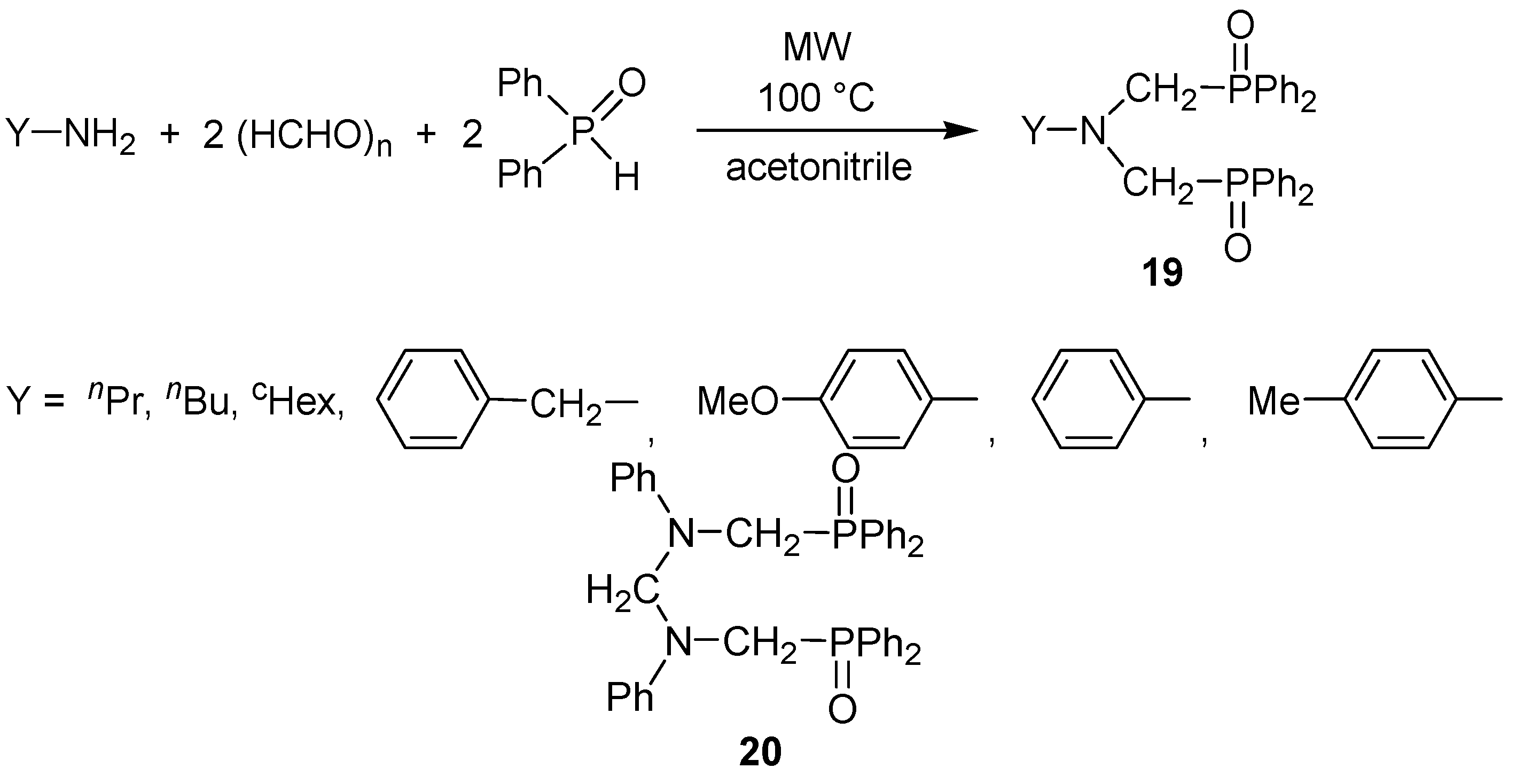
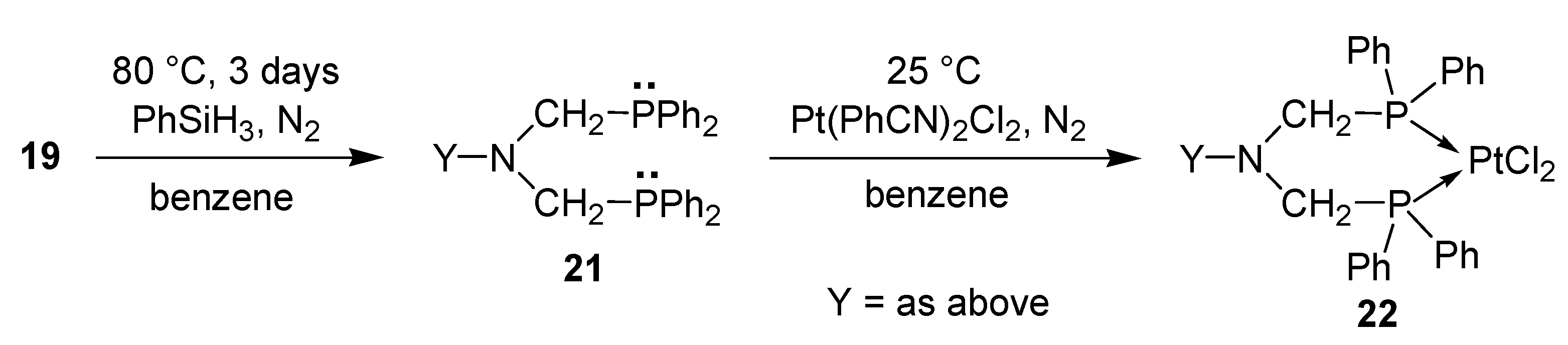
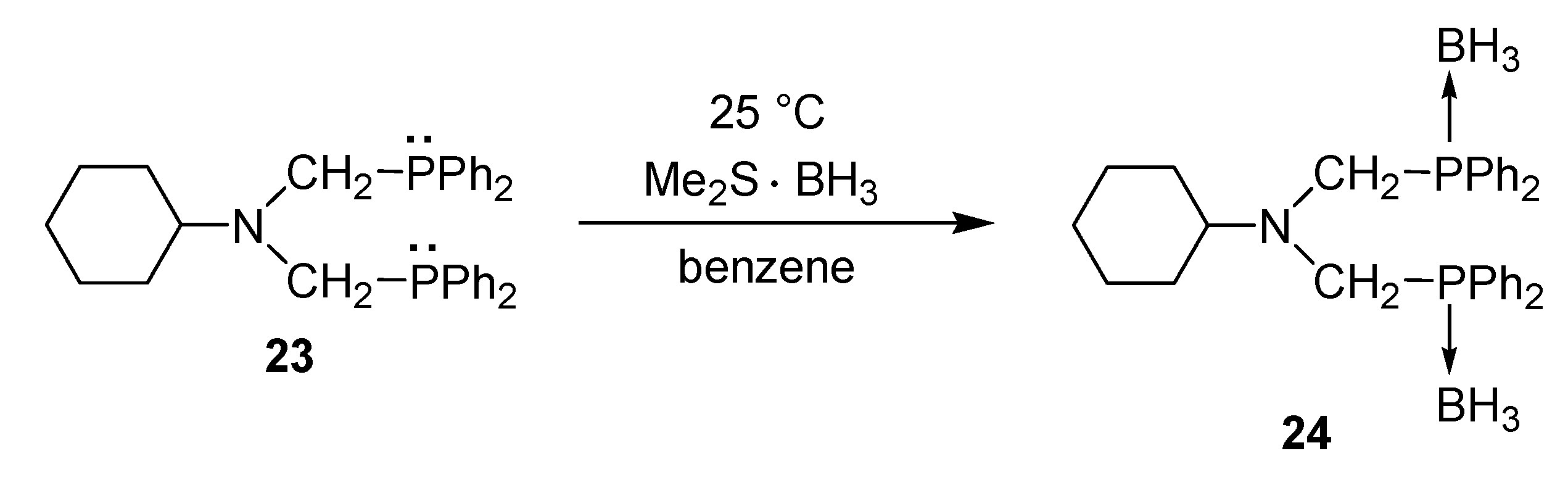

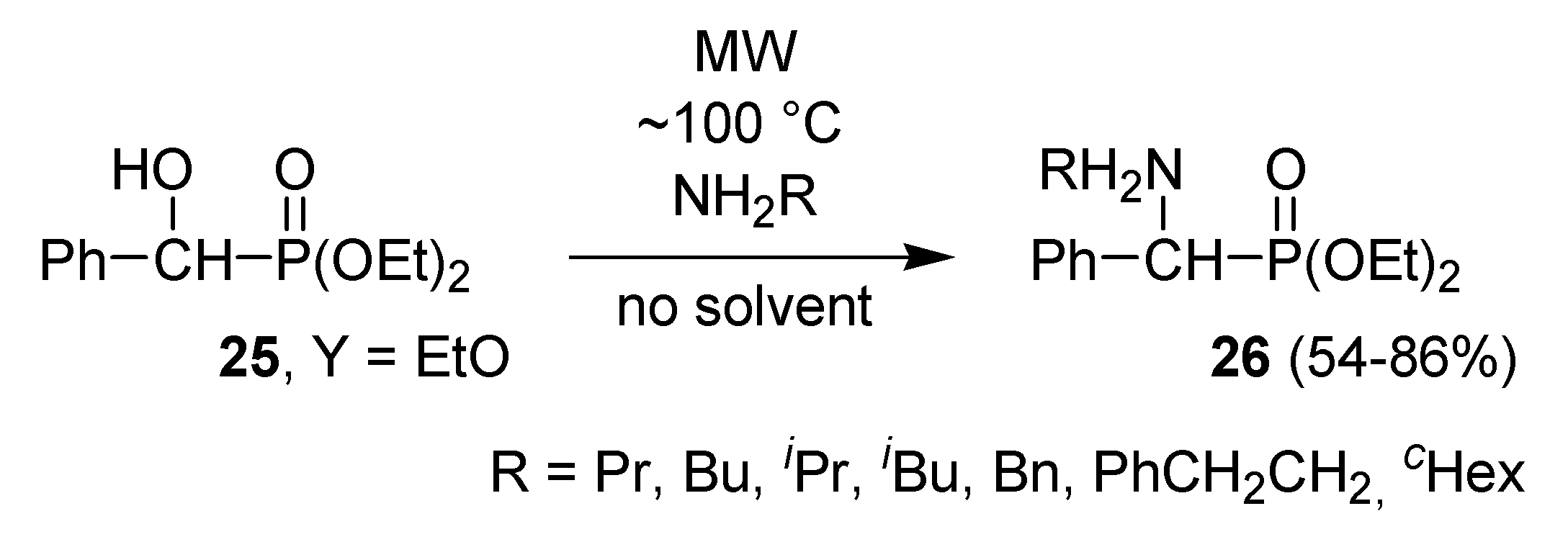
4. Conclusions
Acknowledgments
References
- Kabachnik, M.I.; Medved, T.Y. New synthesis of aminophosphonic acids. Dokl. Akad. Nauk SSSR 1952, 83, 689–692. [Google Scholar]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Matveeva, E.D. Catalytic Kabachnik-Fields reaction: New horizons for old reaction. ARKIVOC 2008, 1–17. [Google Scholar]
- Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Kukhar, V.P.; Hudson, H.R. (Eds.) Wiley: Chichester, UK, 2000.
- Fields, S.C. Synthesis of natural products containing a C-P bond. Tetrahedron 1999, 55, 12237–12272. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anticancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Bird, J.; De Mello, R.C.; Harper, G.P.; Hunter, D.J.; Karran, E.H.; Markwell, R.E.; Miles-Williams, A.J.; Rahman, S.S.; Ward, R.W. Synthesis of novel N-phosphonoalkyl dipeptide inhibitors of human collagenase. J. Med. Chem. 1994, 37, 158–169. [Google Scholar] [CrossRef]
- Liu, W.S.; Rogers, C.J.; Fisher, A.J.; Toney, M.D. Aminophosphonate inhibitors of dialkylglycine decarboxylase: Structural basis for slow binding inhibition. Biochemistry 2002, 41, 12320–12328. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Sienczyk, M.; Oleksyszyn, J. Irreversible inhibition of serine proteases-Design and in vivo activity of diaryl alpha-aminophosphonate derivatives. Curr. Med. Chem. 2009, 16, 1673–1687. [Google Scholar] [CrossRef]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med. Chem. 2003, 46, 2641–2655. [Google Scholar]
- Long, N.; Cai, X.J.; Song, B.A.; Yang, S.; Chen, Z.; Bhadury, P.S.; Hu, D.Y.; Jin, L.H.; Xue, W. Synthesis and antiviral activities of cyanoacrylate derivatives containing an alpha-aminophosphonate moiety. J. Agric. Food Chem. 2008, 56, 5242–5246. [Google Scholar]
- Hu, D.Y.; Wan, Q.Q.; Yang, S.; Song, B.A.; Bhadury, P.S.; Jin, L.H.; Yan, K.; Liu, F.; Chen, Z.; Xue, W. Synthesis and antiviral activities of amide derivatives containing the alpha-aminophosphonate moiety. J. Agric. Food Chem. 2008, 56, 998–1001. [Google Scholar]
- Danila, D.C.; Wang, X.Y.; Hubble, H.; Antipin, I.S.; Pinkhassik, E. Increasing permeability of phospholipid bilayer membranes to alanine with synthetic alpha-aminophosphonate carriers. Bioorg. Med. Chem. Lett. 2008, 18, 2320–2323. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Galkin, V.I. The Kabachnik-Fields reaction: synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857–882. [Google Scholar] [CrossRef]
- Galkin, V.I.; Zvereva, E.R.; Sobanov, A.A.; Galkina, I.V.; Cherkasov, R.A. Kinetics and mechanism of Kabachnik-Fields reaction in dialkylphosphite-benzaldehyde-aniline system. Zhur. Obsch. Khim. 1993, 63, 2224–2227. [Google Scholar]
- Galkina, I.V.; Sobanov, A.A.; Galkin, V.I.; Cherkasov, R.A. Kinetics and mechanism of the Kabachnik-Fields reaction: IV. Salicyaldehyde in the Kabachnik-Fields reaction. Russ. J. Gen. Chem. 1998, 68, 1398–1401. [Google Scholar]
- Matveeva, E.D.; Zefirov, N.S. On the mechanism of the Kabachnik-Fields reaction: Does a mechanism of nucleophilic amination of alpha-hydroxyphosphonates exist? Doklady Chem. 2008, 420, 137–140. [Google Scholar] [CrossRef]
- Galkina, I.V.; Galkin, V.I.; Cherkasov, R.A. Kinetics and the mechanism of Kabachnik-Fields reaction-V. Effect of the nature of hydrophosphoryl compound on the mechanism of Kabachnik-Fields reaction. Zhur. Obsch. Khim. 1998, 68, 1469–1475. [Google Scholar]
- Gancarz, R.; Gancarz, I. Failure of aminophosphonate synthesis due to facile hydroxyphosphonate-phosphate rearrangement. Tetrahedron Lett. 1993, 34, 145–148. [Google Scholar] [CrossRef]
- Gancarz, R. Nucleophilic addition to carbonyl compounds. competition between hard (amine) and soft (phosphite) nucleophile. Tetrahedron 1995, 51, 10627–10632. [Google Scholar]
- Keglevich, G.; Fehérvári, A.; Csontos, I. A study on the Kabachnik-Fields reaction of benzaldehyde, propylamine and diethyl phosphite by in situ Fourier transform (FT) IR spectroscopy. Heteroatom Chem. 2011, 22, 599–604. [Google Scholar] [CrossRef]
- Keglevich, G.; Kiss, N.Z.; Menyhárd, D.; Fehérvári, A.; Csontos, I. A study on the Kabachnik-Fields reaction of benzaldehyde, cyclohexylamine and dialkyl phosphites. Heteroatom Chem. 2012, 23, 171–178. [Google Scholar] [CrossRef]
- Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Microwave-assisted solvent-free and catalyst-free Kabachnik-Fields reactions for α-amino phosphonates. Tetrahedron Lett. 2006, 47, 1125–1127. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A. Eco-friendly accomplishment of the extended Kabachnik-Fields reaction; a solvent- and catalyst-free microwave-assisted synthesis of a-aminophosphonates and a-aminophosphine oxides. Lett. Org. Chem. 2008, 5, 616–622. [Google Scholar] [CrossRef]
- Matveeva, E.D.; Podrugina, T.A.; Tishkovskaya, E.V.; Tomilova, L.G.; Zefirov, N.S. A novel catalytic three-component synthesis (Kabachnick-Fields reaction) of α-aminophosphonates from ketones. Synlett 2003, 2321–2324. [Google Scholar]
- Bhagat, S.; Chakraborti, A.K. An extremely efficient three-component reaction of aldehydes/ketones, amines, and phosphites (Kabachnik-Fields reaction) for the synthesis of a-aminophosphonates catalyzed by magnesium perchlorate. J. Org. Chem. 2007, 72, 1263–1270. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. A facile and highly efficient route to α-amino phosphonates via three-component reactions catalyzed by Mg(ClO4)2 or molecular iodine. Org. Biomol. Chem. 2006, 4, 1663–1666. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Sobhani, S. Metal triflate-catalyzed one-pot synthesis of α-aminophosphonates from carbonyl compounds in the absence of solvent. Synthesis 2004, 2692–2696. [Google Scholar]
- Sun, P.; Hu, Z.; Huang, Z. Gallium triiodide catalyzed organic reaction: a convenient synthesis of α-amino phosphonates. Synth. Commun. 2004, 34, 4293–4299. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A.; Maiti, D.K. In(OTf)(3) catalysed simple one-pot synthesis of α-amino phosphonates. J. Mol. Catal. A 2004, 210, 53–57. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Kaur, T. An efficient one-pot synthesis of alpha-amino phosphonates catalyzed by bismuth nitrate pentahydrate. Synlett 2007, 745–748. [Google Scholar] [CrossRef]
- Zhan, Z.-P.; Li, J.-P. Bismuth(III) chloride-catalyzed three-component coupling: Synthesis of alpha-amino phosphonates. Synth. Commun. 2005, 35, 2501–2508. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Wang, W.-Z.; Xiu, H.-G. A highly efficient catalyst FeCl3 in the synthesis of α-amino phosphonates via three-component reactions. Chin. J. Chem. 2006, 24, 1054–1057. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Wu, J.; Shen, Q.; Chen, H. Facile one-pot synthesis of α-amino phosphonates using lanthanide chloride as catalyst. Heteroatom Chem. 2006, 17, 389–392. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Deng, M.; Shen, Q. One-pot synthesis of α-amino phosphonates using samarium diiodide as a catalyst precursor. Eur. J. Org. Chem. 2003, 4728–4730. [Google Scholar]
- Ravinder, K.; Vijender Reddy, A.; Krishnaiah, P.; Venkataramana, G.; Niranjan Reddy, V.L.; Venkateswarlu, Y. CAN catalyzed one-pot synthesis of α-amino phosphonates from carbonyl compounds. Synth. Commun. 2004, 34, 1677–1683. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. General procedure for the synthesis of α-amino phosphonates from aldehydes and ketones using indium(III) chloride as a catalyst. Org. Lett. 1999, 1, 1141–1143. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.K.; Song, C.E.; Kim, D.-C. Microwave-assisted Kabachnik–Fields reaction in ionic liquid. Bull. Korean Chem. Soc. 2002, 23, 667–668. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.H.; Kang, J.; Lee, J.K. Lanthanide triflate-catalyzed three component synthesis of a-amino phosphonates in ionic liquids. A catalyst reactivity and reusability study. Chem. Commun. 2001, 1698–1699. [Google Scholar]
- Yadav, J.S.; Reddy, B.V.S.; Sreedhar, P. An eco-friendly approach for the synthesis of α-aminophosphonates using ionic liquids. Green Chem. 2002, 4, 436–438. [Google Scholar] [CrossRef]
- Prauda, I.; Greiner, I.; Ludányi, K.; Keglevich, G. Efficient synthesis of phosphono- and phosphinoxidomethylated N-heterocycles under solvent-free microwave conditions. Synth. Commun. 2007, 37, 317–322. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A.; Sipos, M.; Ludányi, K.; Greiner, I. Synthesis of cyclic aminomethylphosphonates and aminomethyl-arylphosphinic acids by an efficient microwave-mediated phospha-Mannich approach. Heteroatom Chem. 2008, 19, 207–210. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A.; Szöllősy, Á.; Drahos, L. Synthesis of bisp(hosphonatomethyl)-, bis(phosphinatomethyl)-, and bis(phosphinoxidomethyl)amines, as well as related ring bis(phosphine) platinum complexes. Synth. Commun. 2011, 41, 2265–2272. [Google Scholar]
- Bálint, E.; Fazekas, E.; Pintér, G.; Szöllősy, Á.; Holczbauer, T.; Czugler, M.; Drahos, L.; Körtvélyesi, T.; Keglevich, G. Synthesis and utilization of the bis(>P(O)CH2)amine derivatives obtained by the double Kabachnik–Fields reaction with cyclohexylamine; Quantum chemical and X-ray study of the related bidentate chelate platinum complexes. Curr. Org. Chem. 2012, 16, 547–554. [Google Scholar]
- Bálint, E.; Fazekas, E.; Pongrácz, P.; Kollár, L.; Drahos, L.; Holczbauer, T.; Czugler, M.; Keglevich, G. N-benzyl and N-aryl bis(phospha-Mannich adducts): Synthesis and catalytic activity of the related bidentate chelate platinum complexes in hydroformylation. J. Organomet. Chem. 2012, 717, 75–82. [Google Scholar]
- Gourdel, Y.; Pelon, P.; Toupet, L.; Le Corre, M. Stereoselective synthesis of new functionalized bisphosphines. Tetrahedron Lett. 1994, 35, 1197–1200. [Google Scholar] [CrossRef]
- Keglevich, G.; Tóth, V. R.; Drahos, L. Microwave-assisted synthesis of α-hydroxy-benzylphosphonates and -benzylphosphine oxides. Heteroatom Chem. 2011, 22, 15–17. [Google Scholar] [CrossRef]
- Grün, A.; Molnár, I.G.; Bertók, B.; Greiner, I.; Keglevich, G. Synthesis of α-hydroxy-methylenebisphosphonates by the microwave-assisted reaction of α-oxophosphonates and dialkyl phosphites under solventless conditions. Heteroatom Chem. 2009, 20, 350–354. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Molnár, I.G.; Greiner, I. Phenyl-, benzyl- and unsymmetrical hydroxy-methylenebisphosphonates as dronic acid ester analogues from α-oxophosphonates by microwave-assisted synthesis. Heteroatom Chem. 2011, 22, 640–648. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Kaszás, A.; Drahos, L.; Mucsi, Z.; Keglevich, G. A neighbouring group effect leading to enhanced nucleophilic substitution of amines at the hindered α-carbon atom of an α-hydroxyphosphonate. Tetrahedron Lett. 2012, 53, 207–209. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821-12835. https://doi.org/10.3390/molecules171112821
Keglevich G, Bálint E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules. 2012; 17(11):12821-12835. https://doi.org/10.3390/molecules171112821
Chicago/Turabian StyleKeglevich, György, and Erika Bálint. 2012. "The Kabachnik–Fields Reaction: Mechanism and Synthetic Use" Molecules 17, no. 11: 12821-12835. https://doi.org/10.3390/molecules171112821