Gamma Irradiation Increases the Antioxidant Properties of Tualang Honey Stored Under Different Conditions
Abstract
:1. Introduction
2. Results
2.1. Polyphenol Content
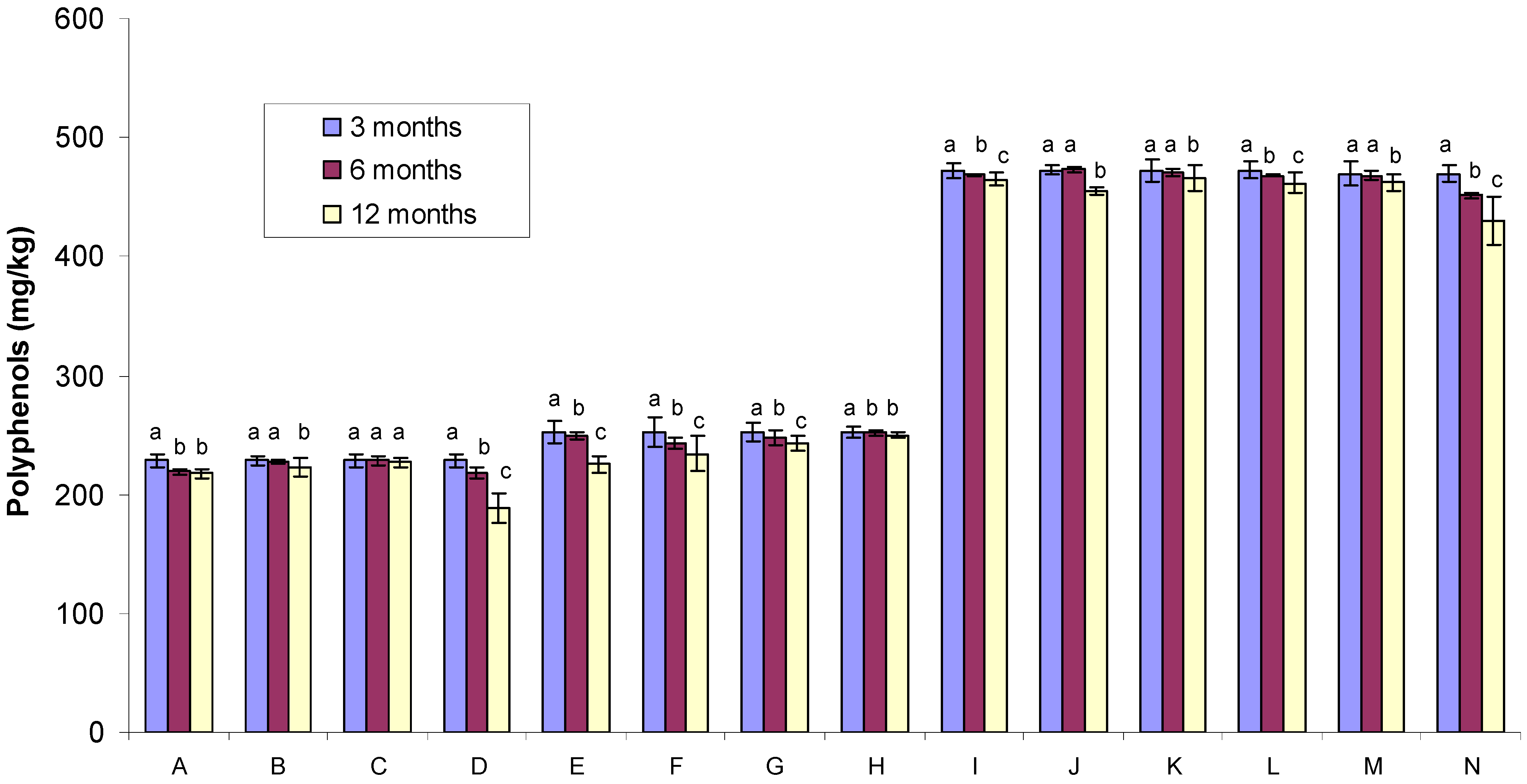
| Sample no. | Gamma irradiated condition | Glass container | Temperature * | Sample code |
|---|---|---|---|---|
| 1 | Non-evaporated and non-gamma irradiated | Dark bottle | Cold room | A |
| 2 | Non-evaporated and non-gamma irradiated | Dark bottle | Room temperature | B |
| 3 | Non-evaporated and non-gamma irradiated | Clear bottle | Room temperature | C |
| 4 | Non-evaporated and non-gamma irradiated | Clear bottle | Cold room | D |
| 5 | Evaporated and non-gamma irradiated | Dark bottle | Cold room | E |
| 6 | Evaporated and non-gamma irradiated | Clear bottle | Cold room | F |
| 7 | Evaporated and non-gamma irradiated | Clear bottle | Room temperature | G |
| 8 | Evaporated and non-gamma irradiated | Dark bottle | Room temperature | H |
| 9 | Evaporated and gamma irradiated | Dark bottle | Room temperature | I |
| 10 | Evaporated and gamma irradiated | Clear bottle | Room temperature | J |
| 11 | Evaporated and gamma irradiated | Dark bottle | Cold room | K |
| 12 | Evaporated and gamma irradiated | Clear bottle | Cold room | L |
| 13 | Sachet (evaporated and gamma irradiated) | Sachet | Room temperature | M |
| 14 | Sachet (evaporated and gamma irradiated) | Sachet | Cold room | N |
2.2. Flavonoid Content
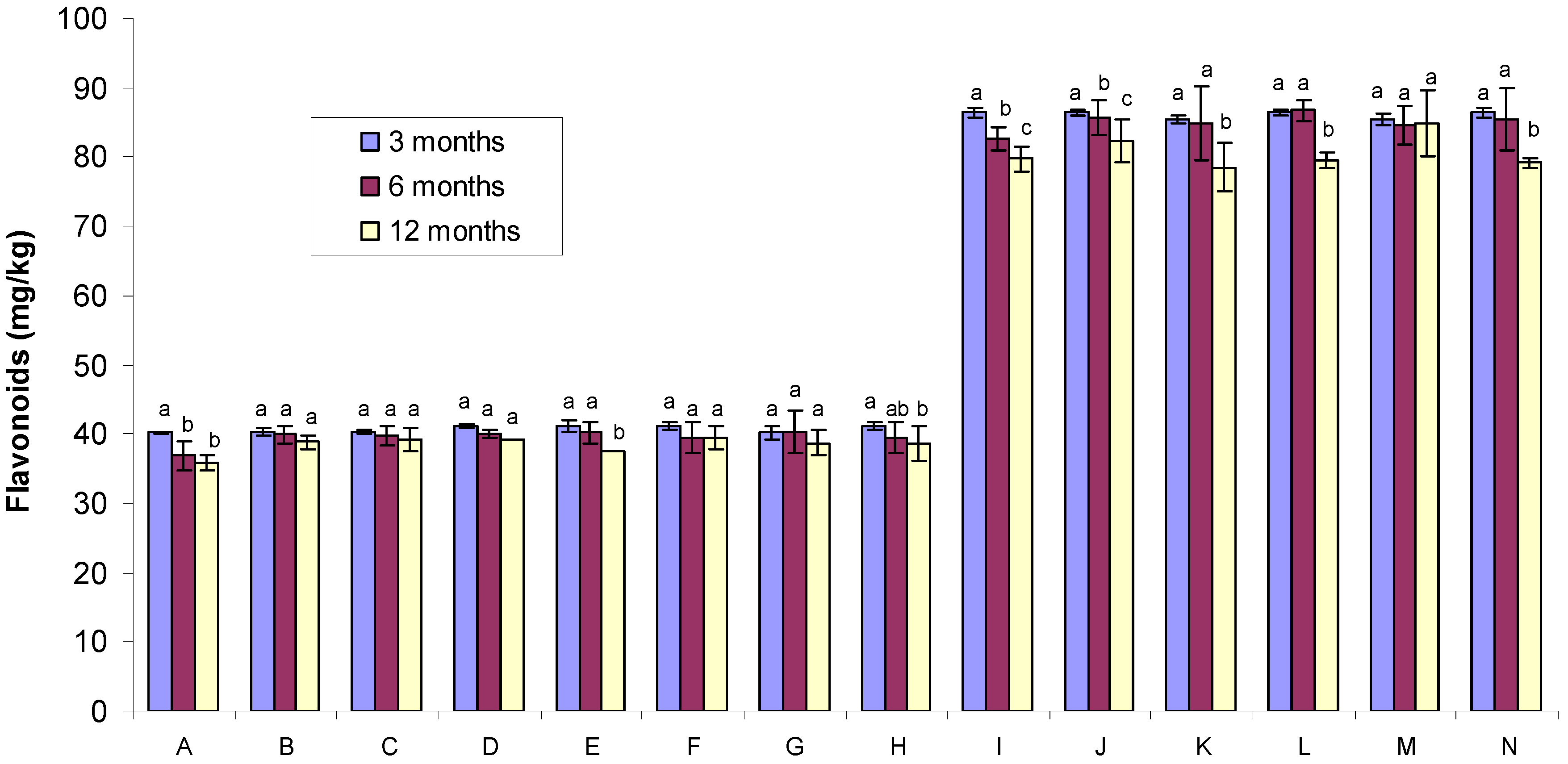
2.3. DPPH Free Radical-Scavenging Activity of Gamma Irradiated Honeys
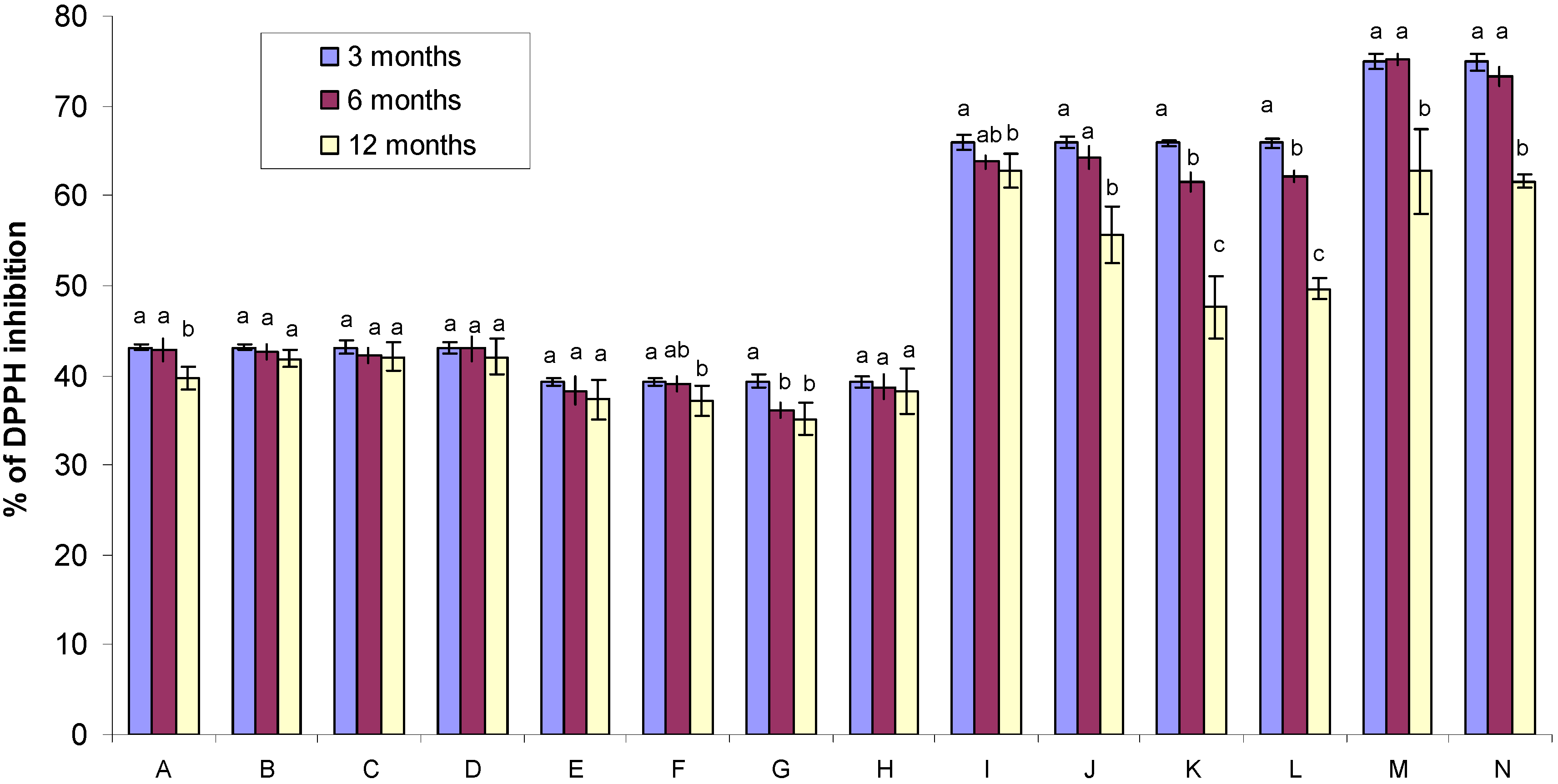

| Polyphenols | Flavonoids | DPPH | |
|---|---|---|---|
| Polyphenols | 1 | 0.98(**) | 0.98(**) |
| Flavonoids | 0.98(**) | 1 | 0.98(**) |
| DPPH | 0.98(**) | 0.98(**) | 1 |
3. Discussion
4. Experimental
4.1. Samples
4.2. Chemicals and Reagents
4.3. Determination of Total Polyphenols
4.4. Determination of Total Flavonoids
4.5. DPPH Free Radical-Scavenging Activity
4.6. Statistical Analyses
5. Conclusions
Acknowledgements
References and Notes
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Irish, J.; Carter, D.A.; Blair, S.E.; Heard, T.A. Antibacterial activity of honey from the Australian stingless bee Trigona carbonaria. Int. J. Antimicrob. Agents 2008, 32, 89–90. [Google Scholar] [CrossRef]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef]
- Temaru, E.; Shimura, S.; Amano, K.; Karasawa, T. Atibacterial activity of honey from stingless honeybees (Hymenoptera; Apidae; Meliponinae). Pol. J. Microbiol. 2007, 56, 281–285. [Google Scholar]
- Wang, X.H.; Andrae, L.; Engeseth, N.J. Antimutagenic effect of various honeys and sugars against Trp-p-1. J. Agric. Food Chem. 2002, 50, 6923–6928. [Google Scholar] [CrossRef]
- Beretta, G.; Orioli, M.; Facino, R.M. Antioxidant and radical scavenging activity of honey in endothelial cell cultures (EA.hy926). Planta Med. 2007, 73, 1182–1189. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El-Hady, F.K. Influence of Honey on the Suppression of Human Low Density Lipoprotein (LDL) Peroxidation (In vitro). Evid. Based Complement. Alternat. Med. 2009, 6, 113–121. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Chimi, H.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, J.P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Honey-Health and Therapeutic Qualities; The National Honey Board: ongmont, CO, USA, 2003. Available online: http://www.nhb.org (accessed on 24 March 2011).
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Poyrazoglu, E.S.; Velioglu, Y.S. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006, 95, 653–657. [Google Scholar] [CrossRef]
- Wang, X.H.; Gheldof, N.; Engeseth, N.J. Effect of processing and storage on antioxidant capacity of honey. J. Food Sci. 2004, 69, 96–101. [Google Scholar]
- Ghazali, F.C. Morphological characterization study of Malaysian honey-A VPSEM, EDX randomised attempt. Ann. Microscopy 2009, 9, 93–102. [Google Scholar]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Kirnpal-Kaur, B.S. The antibacterial properties of Malaysian Tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 34–37. [Google Scholar] [CrossRef]
- Tumin, N.; Halim, N.A.; Shahjahan, M.; Noor Izani, N.J.; Sattar, M.A.; Khan, A.H.; Mohsin, S.S.J. Antibacterial activity of local Malaysian honey. Malays. J. Pharm. Sci. 2005, 3, 1–10. [Google Scholar]
- Mohamed, M.; Sirajudeen, K.N.S.; Swamy, M.; Yaacob, N.S.; Sulaiman, S.A. Studies on the antioxidant properties of Tualang honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 59–63. [Google Scholar]
- White, J.W.J. Honey. Adv. Food Res. 1978, 24, 287–375. [Google Scholar] [CrossRef]
- White, J.W.J. Measuring honey quality, a rational approach. Am. Bee J. 1967, 107, 304–375. [Google Scholar]
- White, J.W.J.; Subers, M.H. Studies on honey inhibine. 3. Effect on heat. J. Apicult. Res. 1964, 3, 45–50. [Google Scholar]
- Sancho, M.T.; Muniategui, S.; Huidobro, J.F.; Lozano, J.S. Aging of honey. J. Agric. Food Chem. 1992, 40, 134–138. [Google Scholar] [CrossRef]
- Bath, P.K.; Singh, N. A comparison between Helianthus annuus and Eucalyptus lanceolatus honey. Food Chem. 1999, 67, 389–397. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef]
- Mohamed, M.; Sirajudeen, K.N.S.; Swamy, M.; Yaacob, N.S.; Sulaiman, S.A. Studies on the antioxidant properties of Tualang honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 59–63. [Google Scholar]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the analytical methods for determining the antioxidant properties of honey: A review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 36–42. [Google Scholar]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6384. [Google Scholar]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009, 113, 568–574. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; GonzaLez-Parma, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant Characterization of Native Monofloral Cuban Honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activiity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, D.H.; Jo, C.; Lee, C.H.; Kim, K.S.; Byun, M.W. Effect of gamma irradiation on the microbiological quality and antioxidant activity of fresh vegetable juice. Food Microbiol. 2006, 23, 372–378. [Google Scholar] [CrossRef]
- Stajner, D.; Milosevic, M.; Popovic, B.M. Irradiation effect on phenolic content, lipid and protein oxidation and scavenger ability of Soybean seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef]
- Jo, C.; Son, J.H.; Lee, H.J.; Byun, M.W. Irradiation application for color removal and purification of green tea leaves extract. Radiat. Phys. Chem. 2003, 66, 179–184. [Google Scholar]
- Khattak, K.F.; Simpson, T.J. Ihasnullah Effect of gamma irradiation on the extraction yield, total phenolic content and free radical-scavenging activity of Nigella sataiva seed. Food Chem. 2008, 110, 967–972. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378–6395. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yashura, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the Tualang honeys are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khalil, M.I.; Sulaiman, S.A.; Alam, N.; Moniruzzaman, M.; Bai’e, S.; Man, C.N.; Jamalullail, S.M.S.; Gan, S.H. Gamma Irradiation Increases the Antioxidant Properties of Tualang Honey Stored Under Different Conditions. Molecules 2012, 17, 674-687. https://doi.org/10.3390/molecules17010674
Khalil MI, Sulaiman SA, Alam N, Moniruzzaman M, Bai’e S, Man CN, Jamalullail SMS, Gan SH. Gamma Irradiation Increases the Antioxidant Properties of Tualang Honey Stored Under Different Conditions. Molecules. 2012; 17(1):674-687. https://doi.org/10.3390/molecules17010674
Chicago/Turabian StyleKhalil, Md. Ibrahim, Siti Amrah Sulaiman, Nadia Alam, Mohammed Moniruzzaman, Saringat Bai’e, Che Nin Man, Syed Mohsin Sahil Jamalullail, and Siew Hua Gan. 2012. "Gamma Irradiation Increases the Antioxidant Properties of Tualang Honey Stored Under Different Conditions" Molecules 17, no. 1: 674-687. https://doi.org/10.3390/molecules17010674




