Influence of Halogen Substituents on the Catalytic Oxidation of 2,4,6-Halogenated Phenols by Fe(III)-Tetrakis(p-hydroxyphenyl) porphyrins and Potassium Monopersulfate
Abstract
:1. Introduction
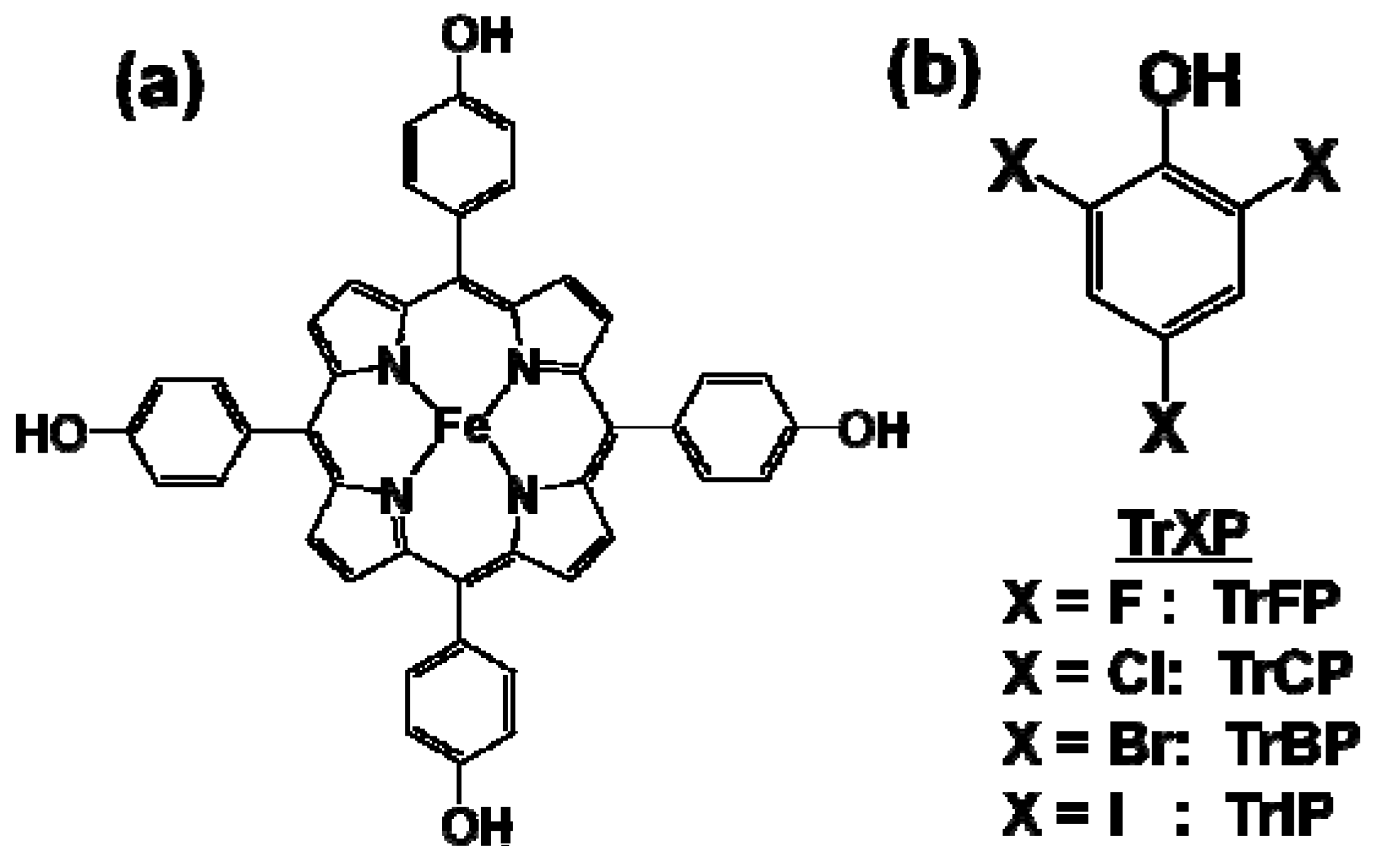
2. Results and Discussion
2.1. Influences of Solution pH on the Degradation and Dehalogenation of TrXPs



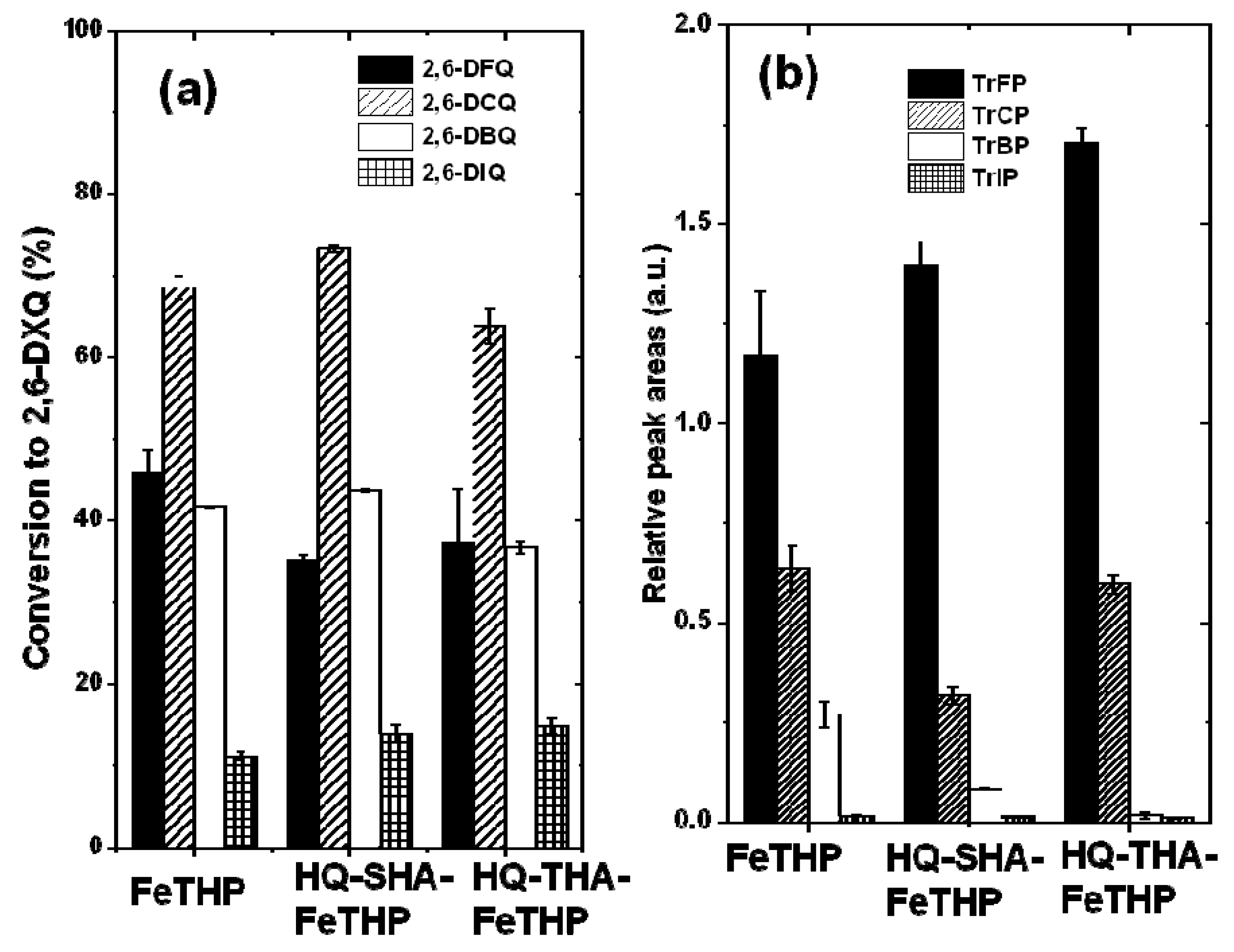
2.2. Turnover Numbers for the Degradation and Dehalogenation of TrXPs

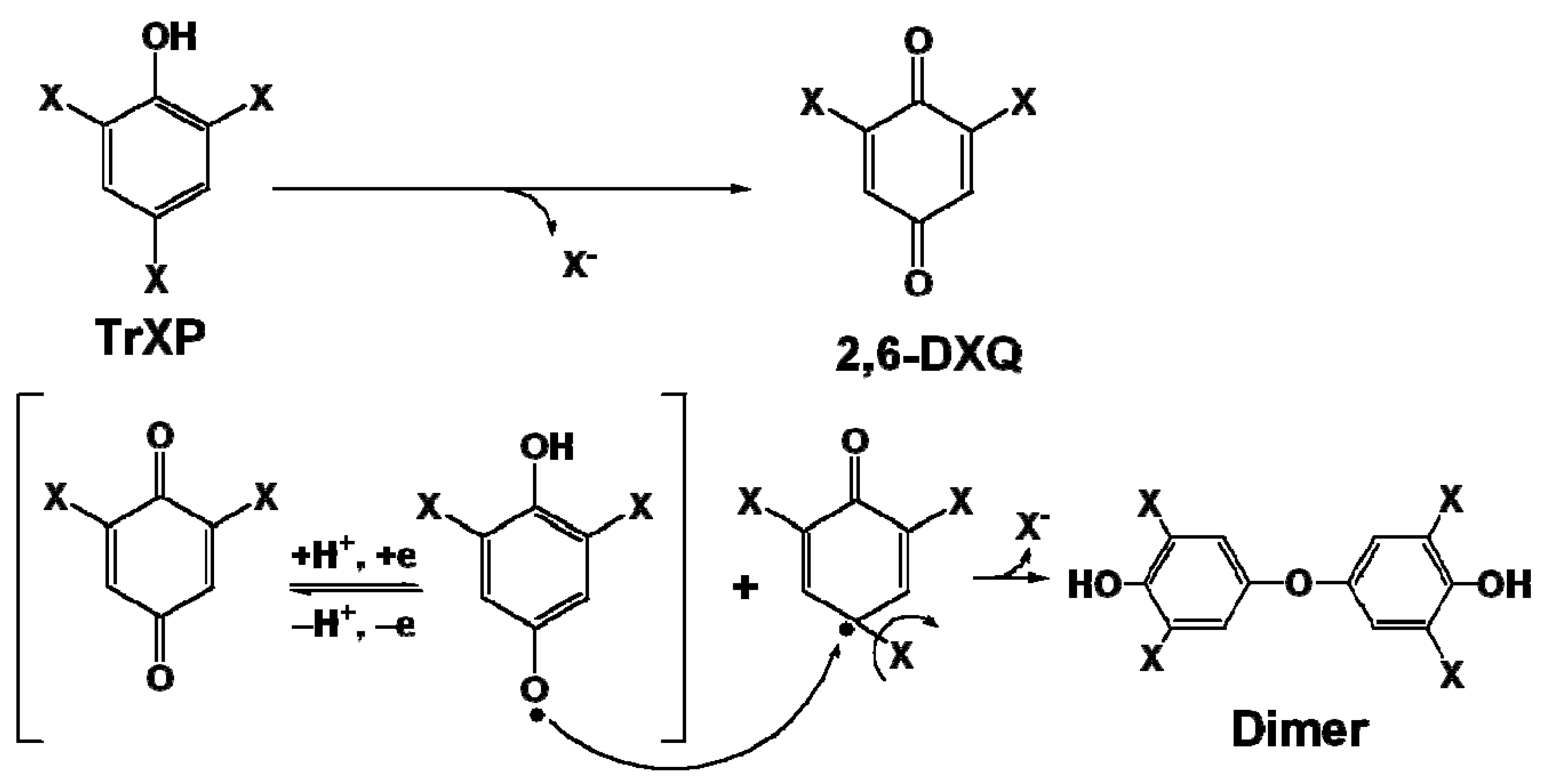
2.3. Oxidation Products



3. Experimental
3.1. Reagents and Materials
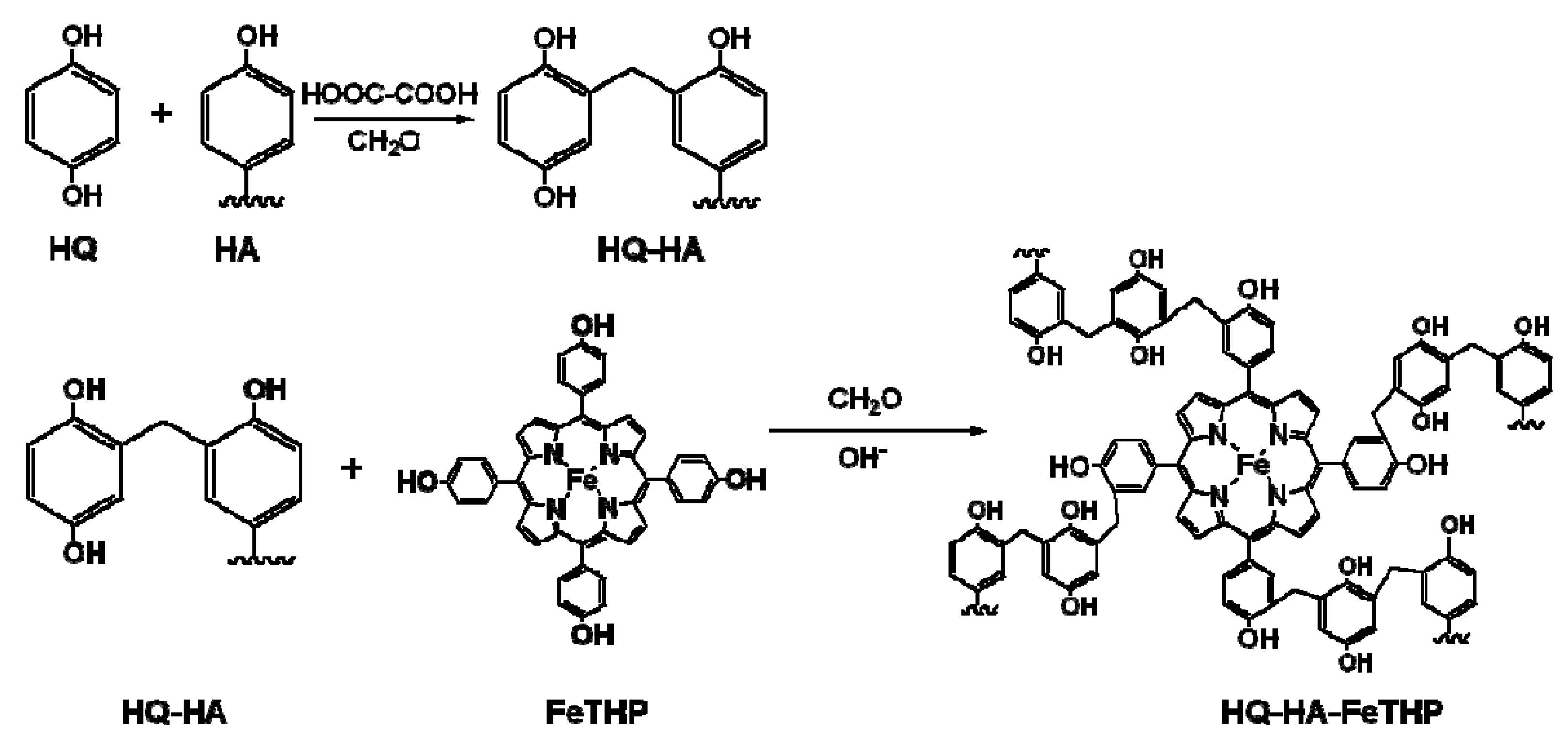
3.2. Synthesis of HQ-HA-FeTHP
| Catalyst | Elemental composition (wt %) | Fe content (μmol mg−1) | |||||
|---|---|---|---|---|---|---|---|
| C | H | N | O | S | ash | ||
| HQ-SHA-FeTHP | 60.00 | 5.30 | 4.31 | 23.99 | 0.39 | 6.01 | 0.55 |
| HQ-THA-FeTHP | 58.66 | 4.71 | 4.02 | 26.15 | 0.94 | 5.52 | 0.60 |
3.3. Tests for Catalytic Activities
4. Conclusions
Acknowledgements
References and Notes
- Osako, M.; Kim, Y.-J.; Sakai, S. Leaching of brominated flame retardants in leachate from landfills in Japan. Chemosphere 2004, 57, 1571–1579. [Google Scholar] [CrossRef]
- Hakk, H.; Letcher, R.J. Metabolism in the toxicokinetics and fate of brominated flame retardants—A review. Environ. Int. 2003, 29, 801–828. [Google Scholar] [CrossRef]
- Keith, L.H.; Telliard, W.A. Priority pollutants I—A perspective view. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metalloporphyrins in Catalytic Oxidations; Dekker: New York, NY, USA, 1994; pp. 325–327. [Google Scholar]
- Shukla, R.S.; Robert, A.; Meunier, B. Kinetic investigations of oxidative degradation of aromatic pollutant 2,4,6-trichlorophenol by an iron-porphyrin complex, a model of ligninase. J. Mol. Catal. A Chem. 1996, 113, 45–49. [Google Scholar] [CrossRef]
- Labat, G.; Seris, J.-L.; Meunier, B. Oxidative degradation of aromatic pollutants by chemical models of ligninase based on porphyrin complexes. Angew. Chem. Ind. Ed. Engl. 1990, 29, 1471–1473. [Google Scholar] [CrossRef]
- Fukushima, M.; Ichikawa, H.; Kawasaki, M.; Sawada, A.; Morimoto, K.; Tatsumi, K. Effects of humic substances on the pattern of oxidation products of pentachlorophenol induced by a biomimetic catalytic system using tetra(p-sulfophenyl)porphineiron(III) and KHSO5. Environ. Sci. Technol. 2003, 37, 386–394. [Google Scholar] [CrossRef]
- Fukushima, M.; Sawada, A.; Kawasaki, M.; Ichikawa, H.; Morimoto, K.; Tatsumi, K.; Aoyama, M. Influence of humic substances on the removal of pentachlorophenol by a biomimetic catalytic system with a water-soluble iron(III)-porphyrin complex. Environ. Sci. Technol. 2003, 37, 386–394. [Google Scholar] [CrossRef]
- Rismayani, S.; Fukushima, M.; Sawada, A.; Ichikawa, H.; Tatsumi, K. Effects of peat humic acids on the catalytic oxidation of pentachlorophenol using metalloporphyrins and metallophthalocyanines. J. Mol. Catal. A Chem. 2004, 217, 13–19. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K. Effect of hydroxypropyl-β-cyclodextrin on the degradation of pentachlorophenol by potassium monopersulfate catalyzed with iron(III)-porphyrin complex. Environ. Sci. Technol. 2005, 39, 9337–9342. [Google Scholar] [CrossRef]
- Fukushima, M.; Sawada, A.; Kawasaki, M.; Tatsumi, K. Removal of pentachlorophenol from contaminated soil by iron(III)-porphyrin complexes and potassium monopersulfate. Toxicol. Environ. Chem. 2003, 85, 39–49. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K. Oxidative degradation of pentachlorophenol in contaminated soil suspensions by potassium monopersulfate catalyzed with a supramolecular catalyst between iron(III)-porphyrin and hydroxypropyl-β-cyclodextrin. J. Hazard. Mater. 2007, 144, 222–228. [Google Scholar] [CrossRef]
- Fukushima, M.; Shigematsu, S.; Nagao, S. Oxidative degradation of 2,4,6-trichlorophenol and pentachlorophenol in contaminated soil suspension using a supramolecular catalyst prepared via formaldehyde polycondensation between tetrakis(hydroxyphenyl)porphineiron(III) and humic acid. J. Environ. Sci. Heal. A 2009, 44, 1088–1097. [Google Scholar]
- Fukushima, M.; Shigematsu, S.; Nagao, S. Degradation of pentachlorophenol in a contaminated soil suspension using hybrid catalysts prepared via urea-formaldehyde polycondensation between tetrakis(hydroxyphenyl)porphineiron(III) and humic acid. Environ. Chem. Lett. 2011, 9, 223–228. [Google Scholar]
- Nappa, M.J.; Tolman, C.A. Steric and electronic control of iron porphyrin catalyzed hydrocarbon oxidations. Inorg. Chem. 1985, 24, 4711–4719. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K. Complex formation of water-soluble iron(III)-porphyrin with humic acids and their effects on the catalytic oxidation of pentachlorophenol. J. Mol. Catal. A Chem. 2006, 245, 178–184. [Google Scholar] [CrossRef]
- Fukushima, M.; Tanabe, Y.; Morimoto, K.; Tatsumi, K. Role of humic acid fraction with higher aromaticity in enhancing the activity of a biomimetic catalyst, tetra(p-sulfonatophenyl)porphineiron(III). Biomacromolecules 2007, 8, 386–391. [Google Scholar] [CrossRef]
- Fukushima, M. Oxidative degradation of pentachlorophenol by an iron(III)-porphyrin catalyst bound to humic acid via formaldehyde polycondensation. J. Mol. Catal. A Chem. 2008, 286, 47–54. [Google Scholar] [CrossRef]
- Fukushima, M.; Shigematsu, S. Introduction of 5,10,15,20- tetrakis(4-hydroxyphenyl)-porphineiron (III) into humic acid via formaldehyde polycondensation. J. Mol. Catal. A Chem. 2008, 293, 103–109. [Google Scholar] [CrossRef]
- Fukushima, M.; Shigematsu, S.; Nagao, S. Influence of humic acid type on the oxidation products of pentachlorophenol using hybrid catalysts prepared by introducing iron(III)-5,10,15,20-tetrakis(p-hydroxyphenyl)porphyrin into hydroquinone-derived humic acids. Chemosphere 2010, 78, 1155–1159. [Google Scholar] [CrossRef]
- Shigematsu, S.; Fukushima, M.; Nagao, S. Oxidative degradation of 2,6-dibromophenol using an anion-exchange resin supported supramolecular catalysts of iron(III)-5,10,15,20-tetrakis(p-hydroxyphenyl)porphyrin bound to humic acid prepared via formaldehyde and urea-formaldehyde polycondensation. J. Environ. Sci. Heal. A 2010, 45, 1536–1542. [Google Scholar]
- Fukushima, M.; Ishida, Y.; Shigematsu, S.; Kuramitz, H.; Nagao, S. Pattern of oxidation products derived from tetrabromobisphenol A in a catalytic system comprised of iron(III)-tetrakis(p-sulfophenyl)porphyrin, KHSO5 and humic acids. Chemosphere 2010, 80, 860–865. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Bortiatynski, J.M.; Minard, R.D.; Dec, J.; Bollag, J.-M. Use of high-resolution 13C NMR to examine the enzymatic covalent binding 13C-labeled 2,4-dichlorophenol to humic substances. Environ. Sci. Technol. 1993, 27, 2098–2103. [Google Scholar] [CrossRef]
- Dec, J.; Bollag, J.-M. Dehalogenation of chlorinated phenols during oxidative coupling. Environ. Sci. Technol. 1994, 28, 484–490. [Google Scholar] [CrossRef]
- Barker, D.; Brimble, M.A.; Do, P.; Turner, P. Addition of silyloxydienes to 2,6-dibromo-1,4-benzoquinone: An approach to highly oxygenated bromonaphthoquinones for the synthesis of thysanone. Tetrahedron 2003, 59, 2441–2449. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fukushima, M.; Mizutani, Y.; Maeno, S.; Zhu, Q.; Kuramitz, H.; Nagao, S. Influence of Halogen Substituents on the Catalytic Oxidation of 2,4,6-Halogenated Phenols by Fe(III)-Tetrakis(p-hydroxyphenyl) porphyrins and Potassium Monopersulfate. Molecules 2012, 17, 48-60. https://doi.org/10.3390/molecules17010048
Fukushima M, Mizutani Y, Maeno S, Zhu Q, Kuramitz H, Nagao S. Influence of Halogen Substituents on the Catalytic Oxidation of 2,4,6-Halogenated Phenols by Fe(III)-Tetrakis(p-hydroxyphenyl) porphyrins and Potassium Monopersulfate. Molecules. 2012; 17(1):48-60. https://doi.org/10.3390/molecules17010048
Chicago/Turabian StyleFukushima, Masami, Yusuke Mizutani, Shouhei Maeno, Qianqian Zhu, Hideki Kuramitz, and Seiya Nagao. 2012. "Influence of Halogen Substituents on the Catalytic Oxidation of 2,4,6-Halogenated Phenols by Fe(III)-Tetrakis(p-hydroxyphenyl) porphyrins and Potassium Monopersulfate" Molecules 17, no. 1: 48-60. https://doi.org/10.3390/molecules17010048





