Metabolite Profiling of Four Major Flavonoids of Herba Epimdii in Zebrafish
Abstract
:1. Introduction
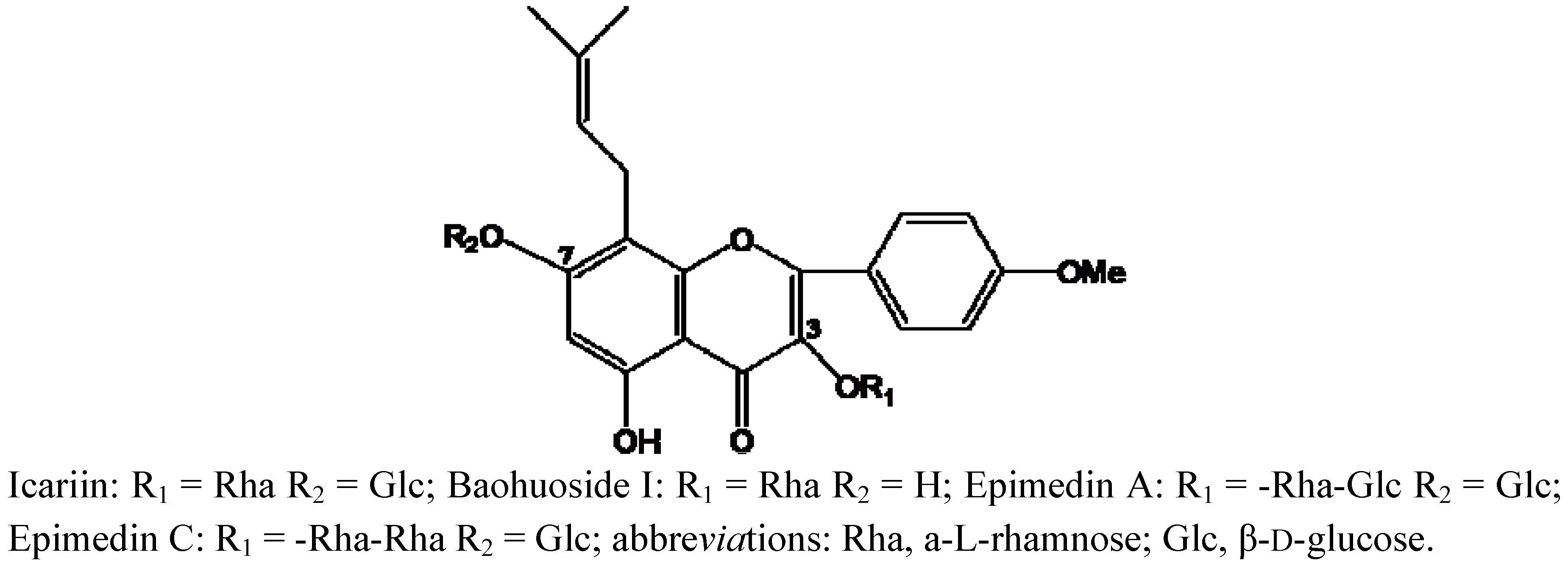
2. Results and Discussion
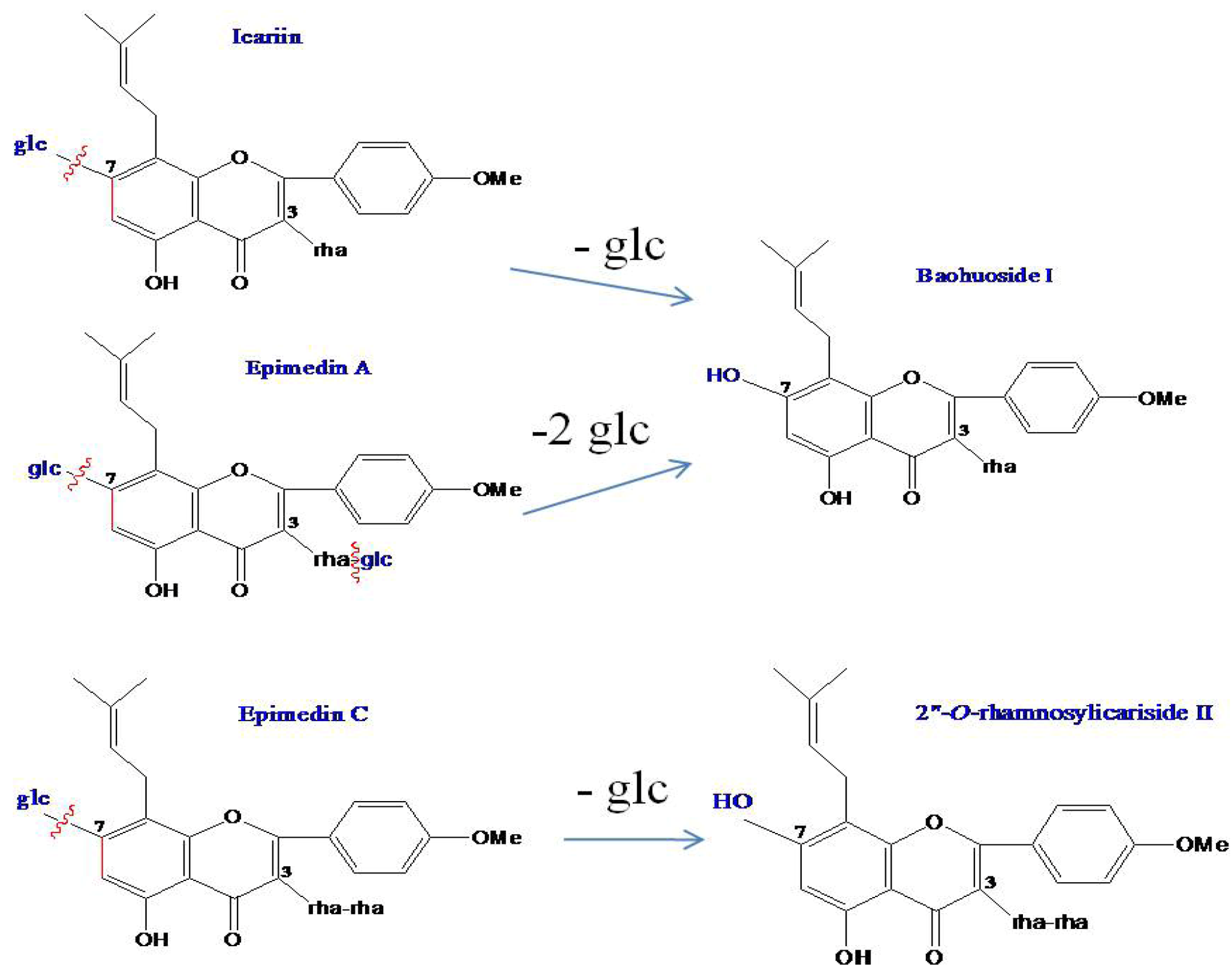
2.1. Analysis of the Metabolic Components of Icariin, Baohuoside I, Epimedin A and Epimedin C after Zebrafish Exposure by HPLC-ESI-MS

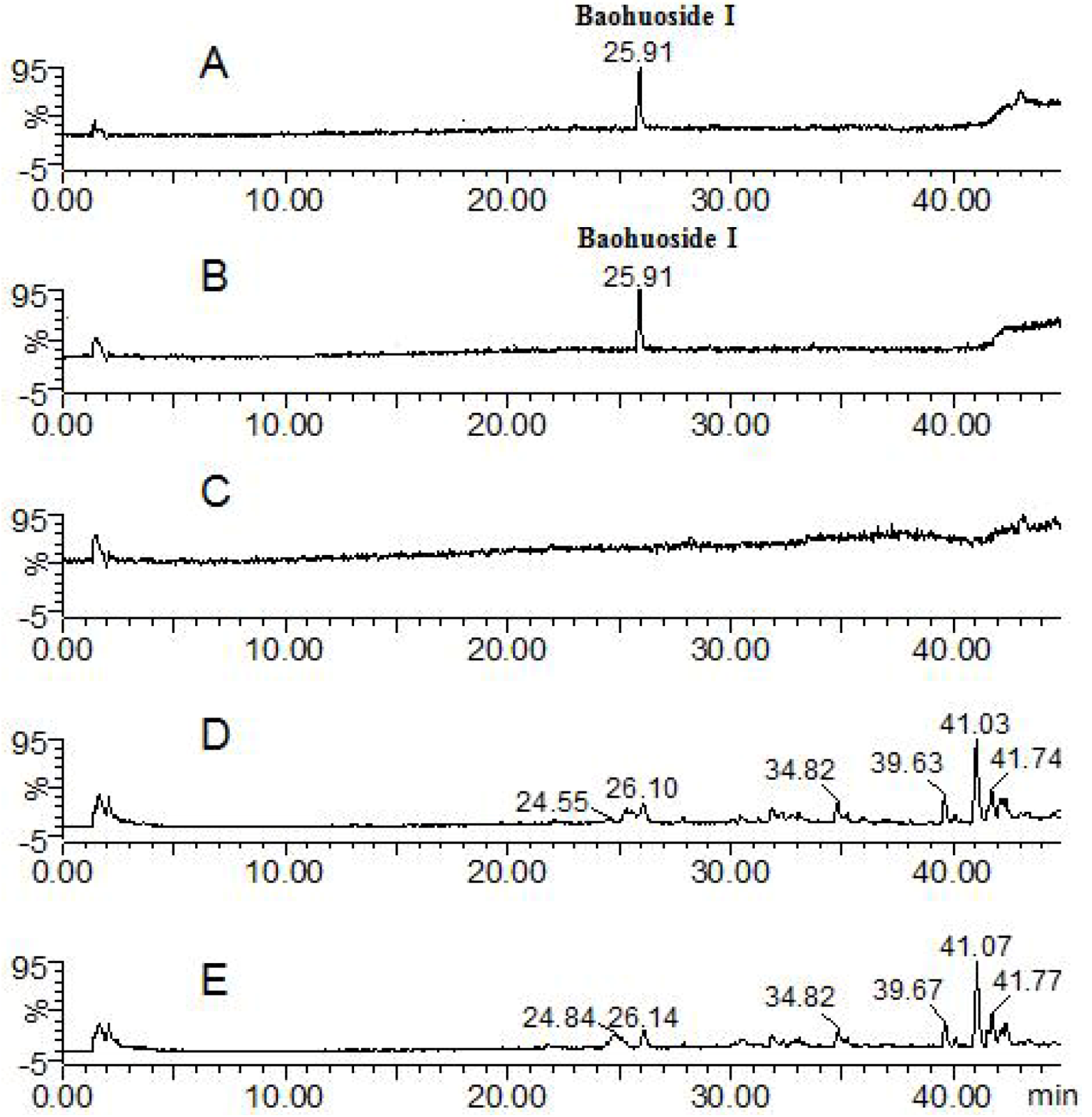
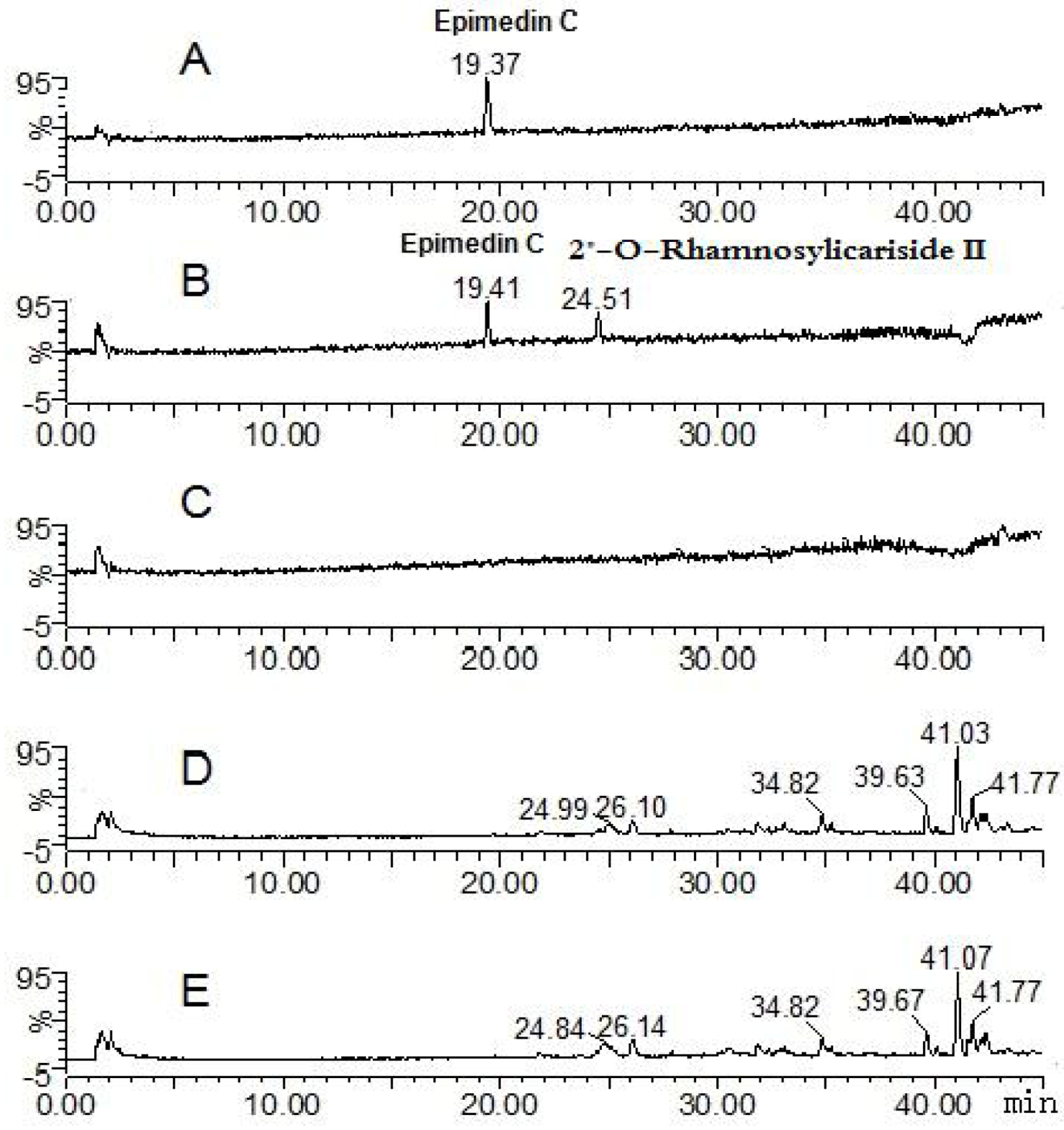
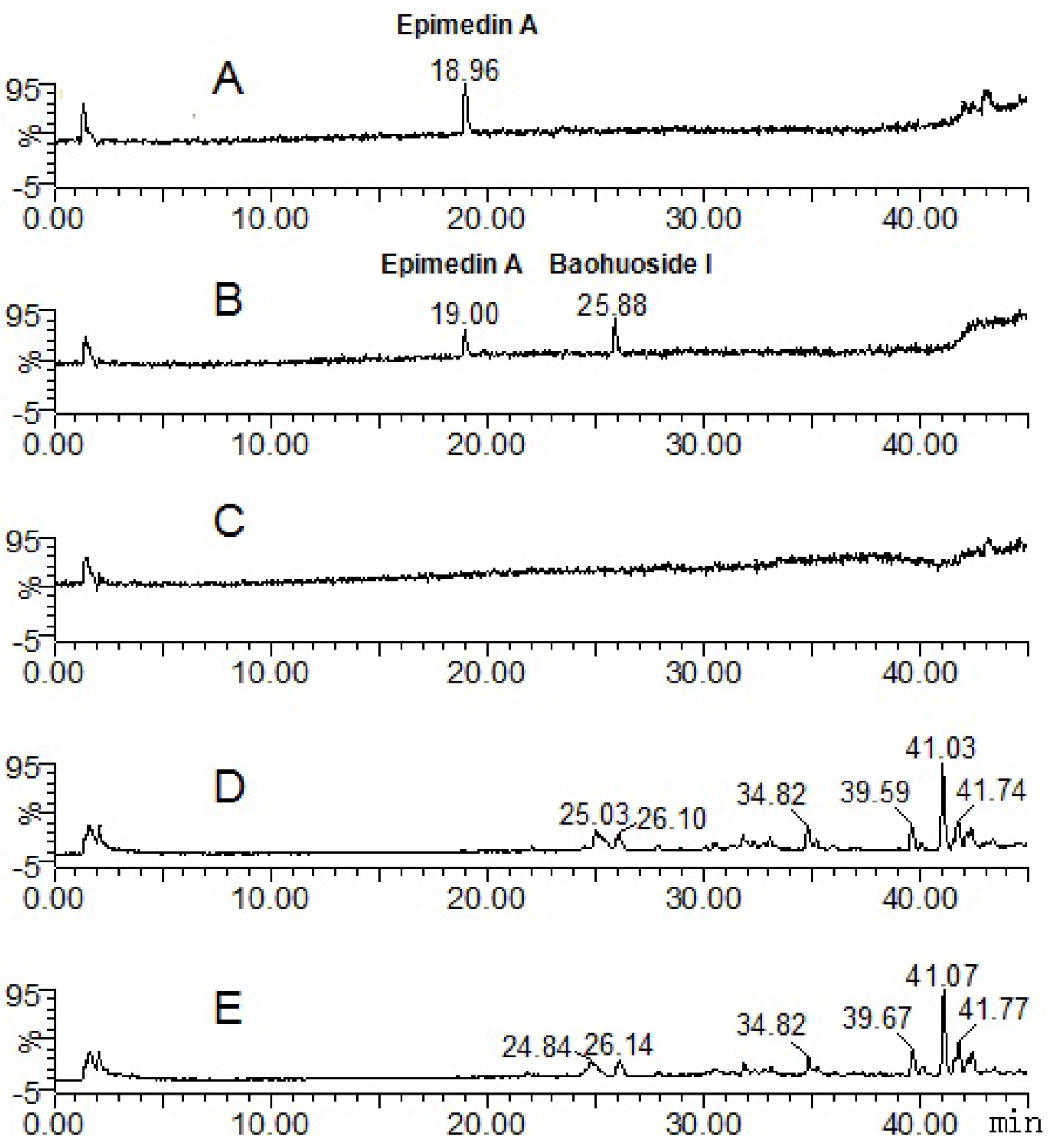
| Compounds | Retention time (min) | quasi-molecular ions | MW | Metabolite presumed | Zebrafish | Current metabolism (references) | ||||
| [M−H]− | [M+HCOO]− | [M+H]+ | [M+Na]+ | solution | body | |||||
| Icariin | 19.81 | 675.26 | 721.70 | 677.88 | 699.58 | 676 | Icariin | + | + | |
| 25.91 | 513.91 | 515.83 | 537.81 | 514 | Baohuoside I | + | + | [10,11] | ||
| Baohuoside I | 25.91 | 513.84 | 515.76 | 537.88 | 514 | Baohuoside I | + | + | [11] | |
| Epimedin A | 19.00 | 837.73 | 883.62 | 839.72 | 861.84 | 838 | Epimedin A | + | ||
| 25.88 | 513.84 | 515.76 | 537.88 | 514 | Baohuoside I | + | ||||
| Epimedin C | 19.41 | 821.79 | 867.68 | 823.71 | 845.49 | 822 | Epimedin C | + | ||
| 24.51 | 659.74 | 661.87 | 683.71 | 660 | 2" -O-rhamnosylicariside II | + | [13] | |||

2.2. Rationality and Advantages of Metabolic Study with Zebrafish Compared to the Existing Model
3. Experimental
3.1. Chemicals and Reagents
3.2. Animals
3.3. Instruments
3.4. Biological Sample Collection
3.5. Sample Preparation
3.6. Analysis Condition
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Wei, Y.J.; Li, P.; Fan, H.W.; Peng, Y.R.; Wang, C.M.; Shu, L.; Jia, X.B. Metabolism study of notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 of Radix Panax notoginseng in zebrafish. Molecules 2011, 16, 6621–6633. [Google Scholar] [CrossRef]
- Wei, Y.J.; Ning, Q.; Jia, X.B.; Gong, Z.N. Thoughts and methods for metabolic study of Chinese materia medica based on zebraf ish model. Chin. Trad. Herb. Drugs 2009, 40, 1009–1011. [Google Scholar]
- Wei, Y.J. Analysis of chemical and metabolic components of fufang danshen prescription. Nanjing China Pharm. Univ. 2007, 21, 797–809. [Google Scholar]
- Wu, C.S.; Guo, B.L.; Sheng, Y.X.; Zhang, J.L. Simultaneous determination of seven flavonoids in epimedium by liquid chromatography-tandem mass spectrometry method. Chin. Chem. Lett. 2008, 19, 329–332. [Google Scholar] [CrossRef]
- Wu, C.S.; Sheng, Y.X.; Zhang, Y.H.; Zhang, J.L.; Guo, B.L. Identification and characterization of active compounds and their metabolites by high-performance liquid chromatography/Fourier transform ion cyclotron resonance mass spectrometry after oral administration of a herbal extract of epimedium koreanum nakai to rats. Rapid Commun. Mass Spectrom. 2008, 22, 2813–2824. [Google Scholar] [CrossRef]
- Wu, C.S.; Zhang, J.L.; Zhou, T.H.; Guo, B.L.; Wang, Y.L.; Hou, J.F. Simultaneous determination of seven flavonoids in dog plasma by ultra-performance liquid chromatography-tandem mass spectrometry and its application to a bioequivalence study of bioactive components in herba epimedii and Er-Xian decoction. J. Pharm. Biomed. Anal. 2011, 54, 186–191. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Lu, X.; Li, C.; Xiong, Z.; Li, F. Simultaneous determination of icariin, icariside II and osthole in rat plasma after oral administration of the extract of gushudan (a chinese compound formulation) by LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 860, 113–120. [Google Scholar] [CrossRef]
- Wong, S.P.; Shen, P.; Lee, L.; Li, J.; Yong, E.L. Pharmacokinetics of prenylflavonoids and correlations with the dynamics of estrogen action in sera following ingestion of a standardized epimedium extract. J. Pharm. Biomed. Anal. 2009, 50, 216–223. [Google Scholar] [CrossRef]
- Shen, P.; Wong, S.P.; Yong, E.L. Sensitive and rapid method to quantify icaritin and desmethylicaritin in human serum using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 857, 47–52. [Google Scholar] [CrossRef]
- Shen, P.; Wong, S.P.; Li, J.; Yong, E.L. Simple and sensitive liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of five Epimedium prenylflavonoids in rat sera. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 71–78. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Xu, W.; Li, N.; Li, H.Y.; Shen, Z.Y.; Zhang, X.M.; Yang, M.; Zhang, W.D.; Zhang, C. LC–MS-MS method for simultaneous determination of icariin and its active metabolite icariside II in human plasma. Chromatographia 2008, 68, 245–250. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.J.; Kano, Y.; Yao, X.S. Metabolism of orally administered icariin in rats. Yao Xue Xue Bao 1999, 24, 222–226. [Google Scholar]
- Xu, W.; Zhang, X.M.; Zhang, Y.P.; Shen, Z.Y.; Zhang, W.D. Metabolism of icariin and icariside II by rat intestinal bacteria in vitro. World Sci. Technol. Modern. Trad. Chin. Med. Mater. Med. 2006, 8, 98–106. [Google Scholar]
- Chen, Y.; Jia, X.B.; Tan, X.B.; Fan, C.Y.; Hu, M. Absorption and metabolism of flavonoids in herba epimedii via rat intestinal perfusion model. China J. Chin. Mater. Med. 2009, 34, 2928–2931. [Google Scholar]
- Chen, Y.; Wang, J.Y.; Jia, X.B.; Tan, X.B.; Hu, M. Role of intestinal hydrolase in the absorption of prenylated flavonoids present in yinyanghuo. Molecules 2011, 16, 1336–1348. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, M.; Fan, L.; Sun, J.; Guo, D. Liquid chromatography-tandem mass spectrometry analysis of metabolites in rats after administration of prenylflavonoids from Epimediums. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1113–1124. [Google Scholar] [CrossRef]
- Liu, T.H.; Wang, B.X.; Wang, Y.; Guo, Y.J.; Wu, L.J.; Nan, L.Y.; Yong, T.N.; Chi, D.Q. Effect of icariin and its metabolites on the production of cytokines by THP-1 cells. Yao Xue Xue Bao 2000, 35, 245–248. [Google Scholar]
- Zhang, T.L.; Huang, J.; Xu, S.J.; Wang, K. Effects of icarrin and its metabolites on the bone metabolism in vitro. J. Capital Univ. Med. Sci. 2005, 26, 37. [Google Scholar]
- Lv, L.; Zhang, H.; Zhao, L.; Jia, J.; Li, Y.Y.; Zhang, G.Q. RRLC-TOF/MS in rapid identification of 43 chemical constituents of epimedium. Acad. J. Sec. Mili. Med. Univ. 2011, 32, 306–310. [Google Scholar]
- Zhao, H.Y.; Sun, J.H.; Fan, M.X.; Fan, L.; Zhou, L.; Li, Z.; Han, J.; Wang, B.R.; Guo, D.A. Analysis of phenolic compounds in epimedium plants using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1190, 157–181. [Google Scholar]
- Wang, Y.Q.; Guo, Z.M.; Jin, Y.; Zhang, X.L.; Wang, L.; Xue, X.Y.; Liang, X.M. Identification of prenyl flavonoid glycosides and phenolic acids in epimedium koreanum nakai by Q-TOF-MS combined with selective enrichment on “click oligo (ethylene glycol) ” column. J. Pharm. Biomed. Anal. 2010, 51, 606–616. [Google Scholar] [CrossRef]
- Li, Z.H.; Alex, D.; Siu, S.O.; Chu, I.K.; Renn, J.; Winkler, C.; Lou, S.; Tsui, S.K.; Zhao, H.Y.; Yan, W.R.; Mahady, G.B.; Li, G.H.; Kwan, Y.W.; Wang, Y.T.; Lee, S.M. Combined in vivo imaging and omics approaches reveal metabolism of icaritin and its glycosides in zebrafish larvae. Mol. Biosyst. 2011, 7, 2128–2138. [Google Scholar]
- Sample Availability: Samples of the compounds icariin, baohuoside I, epimedin A and epimedin C are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, Y.; Li, P.; Fan, H.; Sun, E.; Wang, C.; Shu, L.; Liu, W.; Xue, X.; Qian, Q.; Jia, X. Metabolite Profiling of Four Major Flavonoids of Herba Epimdii in Zebrafish. Molecules 2012, 17, 420-432. https://doi.org/10.3390/molecules17010420
Wei Y, Li P, Fan H, Sun E, Wang C, Shu L, Liu W, Xue X, Qian Q, Jia X. Metabolite Profiling of Four Major Flavonoids of Herba Epimdii in Zebrafish. Molecules. 2012; 17(1):420-432. https://doi.org/10.3390/molecules17010420
Chicago/Turabian StyleWei, Yingjie, Ping Li, Hongwei Fan, E Sun, Changmei Wang, Luan Shu, Wei Liu, Xiaolu Xue, Qian Qian, and Xiaobin Jia. 2012. "Metabolite Profiling of Four Major Flavonoids of Herba Epimdii in Zebrafish" Molecules 17, no. 1: 420-432. https://doi.org/10.3390/molecules17010420
APA StyleWei, Y., Li, P., Fan, H., Sun, E., Wang, C., Shu, L., Liu, W., Xue, X., Qian, Q., & Jia, X. (2012). Metabolite Profiling of Four Major Flavonoids of Herba Epimdii in Zebrafish. Molecules, 17(1), 420-432. https://doi.org/10.3390/molecules17010420




