Variations of Antioxidant Characteristics and Mineral Contents in Pulp and Peel of Different Apple (Malus domestica Borkh.) Cultivars from Pakistan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extract Yields, Total Phenolic and Total Flavonoid Contents
| Variety | TPC (mg gallic acid equivalent/100 g dry weight) | TFC (mg catechin equivalent/100 g dry weight) | ||
|---|---|---|---|---|
| Peel | Pulp | Peel | Pulp | |
| Golden Delicious | 2102.4 ± 44.1 b | 1298.2 ± 26.8 a | 1501.5 ± 31.3 a | 816.3 ± 16.9 a |
| Red Delicious | 2587.9 ± 50.6 a | 1475.5 ± 29.9 a | 1816.4 ± 36.1 a | 930.2 ± 19.9 a |
| Kashmiri Amri | 2097.1 ± 43.4 b | 1185.2 ± 24.7 ab | 1398.4 ± 27.9 ab | 789.3 ± 15.8 a |
| Kala Kulu | 2274.8 ± 49.4 b | 1388.4 ± 26.1 a | 1694.6 ± 37.2 a | 999.3 ± 17.7 a |
| Sky Spur | 1907.5 ± 38.9 b | 1201.2 ± 24.1 a | 1214.3 ± 24.1 b | 711.8 ± 21.2 ab |
| Mean | 2193.9 ± 43.9 a | 1309.7 ± 27.4 b | 1525.0 ± 31.9 a | 849.4 ± 17.9 b |
2.2. Reducing Power of Apple Extract
| Variety | Peel | Pulp |
|---|---|---|
| Golden Delicious | 2.66 ± 0.05 aa | 1.60 ± 0.04 ba |
| Red Delicious | 2.89 ± 0.04 aa | 1.73 ± 0.04 ba |
| Kashmiri Amri | 2.60 ± 0.06 aa | 1.37 ± 0.02 ba |
| Kala Kulu | 2.69 ± 0.07 aa | 1.69 ± 0.03 ba |
| Sky Spur | 2.54 ± 0.06 aa | 1.49 ± 0.03 ba |
2.3. DPPH Radical Scavenging Activity

2.4. Antioxidant Activity of Apple Peel and Pulp Extracts in Linoleic Acid Peroxidation System
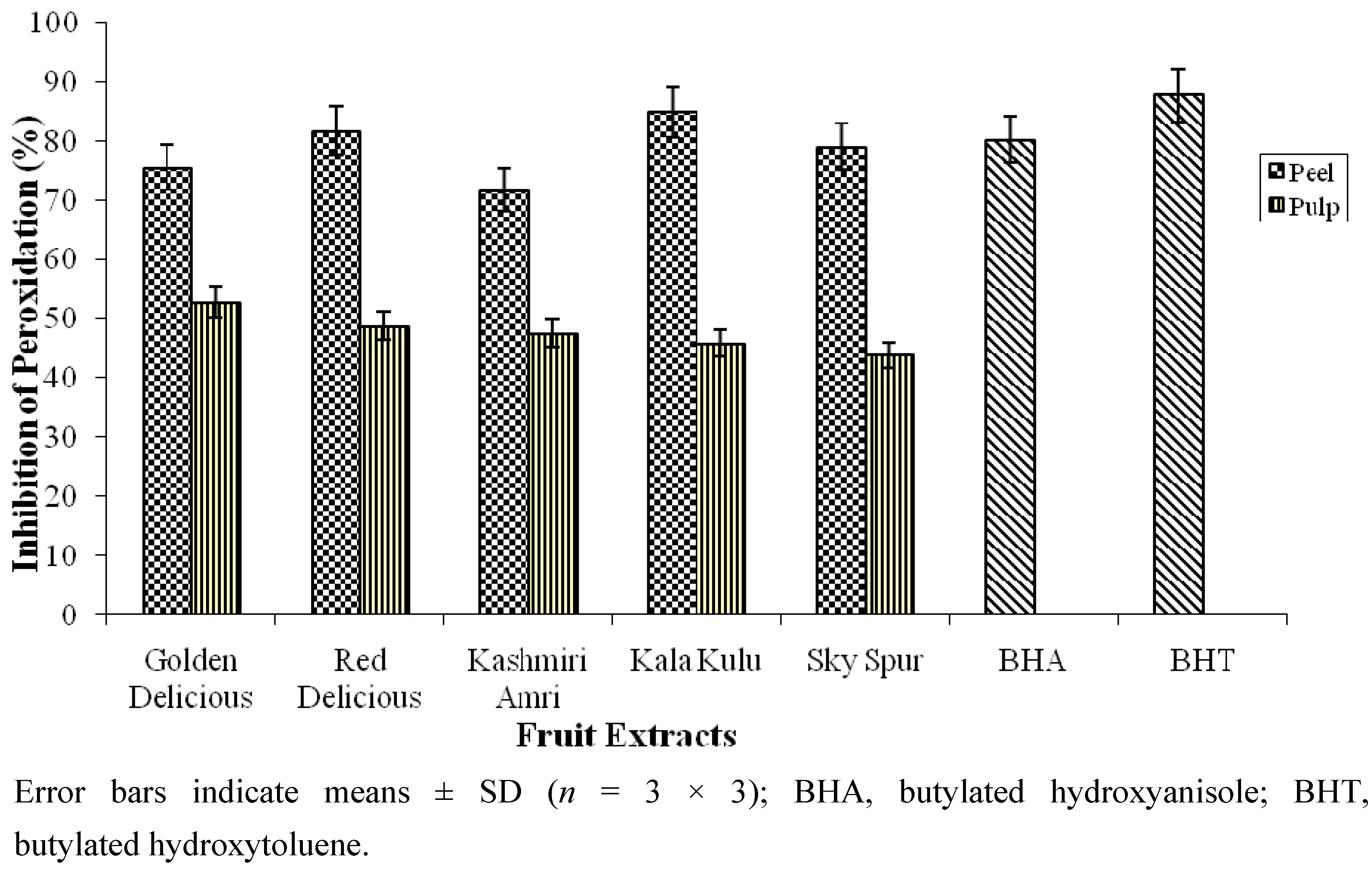
2.5. Minerals
| Variety | Part used | Mineral Contents (mg/100 g dry wt) | ||
|---|---|---|---|---|
| K | Ca | Mg | ||
| Golden Delicious | Peel | 980.9 ± 21.4 a | 61.2 ± 1.32 a | 57.5 ± 1.18 a |
| Pulp | 790.1 ± 17.9 bc | 36.7 ± 0.89 b | 23.9 ± 0.49 b | |
| Red Delicious | Peel | 909.7 ± 17.3 b | 72.1 ±1.47 a | 65.9 ± 1.29 a |
| Pulp | 693.8 ± 13.9 c | 48.9 ± 0.99 b | 34.8 ± 0.73 c | |
| Kashmir Amri | Peel | 724.9 ± 15.5 c | 44.7 ± 0.92 b | 35.7 ± 0.69 c |
| Pulp | 490.1 ± 11.2 d | 22.9 ± 0.67 d | 18.5 ± 0.37 bd | |
| Kala Kulu | Peel | 833.8 ± 17.7 b | 52.2 ± 1.12 ab | 55.8 ± 1.21 ab |
| Pulp | 550.7 ± 12.9 d | 30.5 ± 0.69 bc | 24.9 ± 0.49 d | |
| Sky Spur | Peel | 695.3 ± 15.1 c | 35.6 ± 0.78 bc | 41.9 ± 0.87 c |
| Pulp | 443.6 ± 11.7 de | 19.8 ± 0.41 d | 15.6 ± 0.35 d | |
| Na | Fe | Zn | ||
| Golden Delicious | Peel | 7.3 ± 0.21 b | 2.4 ± 0.04 a | 1.2 ± 0.04 a |
| Pulp | 10.8 ± 0.29 a | 2.1 ± 0.02 a | 0.9 ± 0.01 a | |
| Red Delicious | Peel | 4.7 ± 0.18 c | 1.8 ± 0.04 ab | 0.9 ± 0.06 a |
| Pulp | 9.1 ± 0.17 a | 1.4 ± 0.05 b | 0.7 ± 0.03 b | |
| Kashmir Amri | Peel | 2.9 ± 0.54 cd | 1.6 ± 0.05 b | 0.8 ± 0.05 ab |
| Pulp | 5.3 ± 0.16 c | 1.1 ± 0.02 c | 0.5 ± 0.03 b | |
| Kala Kulu | Peel | 5.9 ± 0.13 c | 2.2 ± 0.04 a | 1.0 ± 0.04 a |
| Pulp | 8.2 ± 0.17 ab | 1.7 ± 0.03 b | 0.8 ± 0.03 ab | |
| Sky Spur | Peel | 7.0 ± 0.11 b | 1.2 ± 0.02 bc | 0.4 ± 0.02 bc |
| Pulp | 10.2 ± 0.09 a | 0.8 ± 0.13 c | 0.2 ± 0.03 c | |
2.6. Correlations among the Results Obtained from Different Antioxidant Assays
| Peel | Variable | TPC | TFC | %Inhibition | DPPH | Reducing power |
| TPC | 1 | |||||
| TFC | 0.952 *** | 1 | ||||
| %Inhibition | 0.480 ns | 0.561 ns | 1 | |||
| DPPH | 0.981 *** | 0.965 *** | 0.583 ns | 1 | ||
| Reducing power | 0.976 *** | 0.912 *** | 0.435 ns | 0.978 *** | 1 | |
| Pulp | TPC | 1 | ||||
| TFC | 0.846 ** | 1 | ||||
| %Inhibition | 0.245 ns | 0.116 ns | 1 | |||
| DPPH | 0.969 *** | 0.744 * | 0.178 ns | 1 | ||
| Reducing power | 0.915 *** | 0.690 * | 0.228 ns | 0.845 ** | 1 |
3. Experimental
3.1. Samples
3.2. Chemicals and Reagents
3.3. Sample Preparation
3.4. Dry Matter Determination
3.5. Antioxidant Activity of Fruits
3.5.1. Extraction
3.5.2. Determination of Extract Yield
3.5.3. Determination of Total Phenolics Content (TPC)
3.5.4. Determination of Total Flavonoid Contents (TFC)
3.5.5. DPPH.Scavenging Assay
3.5.6. Determination of Antioxidant Activity in Linoleic Acid System
3.5.7. Determination of Reducing Power
3.6. Mineral Composition
3.6.1. Preparation of Samples for Mineral Analysis
3.6.2. Preparation of Standards and Analysis of Samples
3.7. Statistical Analysis
4. Conclusions
Conflict of Interest
Acknowledgment
References and Notes
- Abrosca, B.D.; Pacifico, S.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. Limoncella apple, an Italian apple cultivar: phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; Mcdougall, G.J. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009, 67, S145–S150. [Google Scholar] [CrossRef]
- Alberto, M.R.; Rinsdahl-Canavosio, M.A.; Manca de Nadra, M.C. Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Electron. J. Biotechnol. 2006, 9. ISSN: 0717-3458. [Google Scholar]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Liu, R.H. Triterpenoids isolated from apple peels have potent anti-proliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Leontowicz, M.; Gorinstein, S.; Leontowicz, H.; Krzeminski, R.; Lojek, A.; Katrich, E.; Ciz, M.; Martin-Belloso, O.; Soliva-Fortuny, R.; Haruenkit, R.; et al. Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed cholesterol-containing diets. J. Agric. Food Chem. 2003, 51, 5780–5785. [Google Scholar] [CrossRef]
- Lata, B. Relationship between apple peel and the whole fruit antioxidant content: Year and cultivar variation. J. Agric. Food Chem. 2007, 55, 663–671. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Mangas, J.J.; Rodriguez, R.; Suarez, B.; Picinelli, A.; Dapena, E. Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J. Agric. Food Chem. 1999, 47, 4046–4052. [Google Scholar] [CrossRef]
- Podesedeic, A.; Wilska-Jeska, J.; Anders, B.; Markowski, J. Compositional characterization of some apple varieties. Eur. Food Res. Technol. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Weisz, M.; Trakhtenberg, S.; Martın-Belloso, O. Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples. J. Agric. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef]
- Siddiqui, B.N.; Muhammad, S.; Malik, N.H. Effect of socio-economic aspects on the awareness and adoption of recommended horticultural practices by apple growers in Baluchistan, Pakistan. Pak. J. Agric. Sci. 2006, 43, 1–2. [Google Scholar]
- Mukhtar, A.; Gilani, H.; Bhatty, N. Some nutritional and microbiological aspects of apples of common varieties available for household consumption. J. Anim. Plant Sci. 2010, 20, 253–257. [Google Scholar]
- Muhammad, A.; Ayub, M.; Zeb, A.; Durrani, Y.; Ullah, J.; Afridi, S.U.R. Physicochemical analysis of apple pulp from Mashaday variety during storage. Agric. Biol. J. N. Am. 2011, 2, 192–196. [Google Scholar]
- Abid, M. Effect of Citric Acid with Lactic Acid on the Quality and Sensory Characteristics of Apple Drink. M.Sc. Thesis, University of Agriculture, Faisalabad, Pakistan, 2005. [Google Scholar]
- Agricultural Statistic of Pakistan 2006-2007; Government of Pakistan, Ministry of Food, Agriculture and Livestock, Economic Wing: Islamabad, Pakistan; p. 92. 2006-2007.
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Amboni, R.D.D.M.C.; Denardi, F.; Fett, R. Physico-chemical and antioxidant properties of six apple cultivars (Malus domestica Borkh) grown in southern Brazil. Sci. Hortic. 2009, 122, 421–425. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Michailidis, Z.; Pantelidis, G. Peel and flesh antioxidant content and harvest quality characteristics of seven apple cultivars. Sci. Hortic. 2008, 115, 149–153. [Google Scholar]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Pan, Y.; He, C.; Wang, H.; Ji, X.; Wang, K.; Liu, P. Antioxidant activity of microwave-assisted extract of Buddleia officinalis and its major active component. Food Chem. 2010, 121, 497–502. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Zhao, M.; Sun, J.; Wei, X.; Jiang, Y. Effects of high pressure or ultrasonic treatment on extraction yield and antioxidant activity of pericarp tissues of longan fruit. J. Food Biochem. 2010, 34, 838–855. [Google Scholar]
- Ozturk, M.; Ozturk, F.A.; Duru, M.E.; Topcu, G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007, 103, 623–630. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.; Saura-Calixto, F. Free radical scavenging capacity of selected red rosé and white wines. J. Sci. Food Agric. 1999, 79, 1301–1304. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.J.; Yu, L.L. Effect of postharvest treatment and heat stress on availability of wheat antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Bui, H. Biodegradation of mixed phenolic compounds by a microbial association of Aspergillus awamori and Thermoascus aurantiacus. Electron. J. Environ. Agric. Food Chem. 2008, 7, 2625–2633. [Google Scholar]
- Durrani, Y.; Ayub, M.; Muhammad, A.; Ali, A. Pysicochemical response of apple pulp to chemical preservatives and antioxidant during storage. Int.J. Food Saf. 2010, 12, 20–28. [Google Scholar]
- Ismail, F.; Anjum, M.R.; Mamon, A.N.; Kazi, T.G. Trace metal contents of vegetables and fruits of Hyderabad retail market. Pak. J. Nutr. 2011, 10, 365–372. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Kumari, M.; Gupta, S.; Lakshmi, A.; Prakash, J. Iron bioavailability in green leafy vegetables cooked in different utensils. Food Chem. 2004, 86, 217–222. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Compositional characteristics of fruits of several apple (Malus domestica) cultivar. Not. Bot. Hort. Agrobot. Cluj 2010, 38, 228–233. [Google Scholar]
- Ekholm, P.; Reinivuo, H.; Mattila, P. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Compost. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- Leterme, P.; Buldgen, A.; Estrada, F.; Londono, A.M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006, 95, 644–652. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Roble, C.; Brunton, N. A survey of Irish fruit and vegetable waste and by-product as a source of polyphenolic antioxidants. Food Chem. 2009, 116, 202–207. [Google Scholar] [CrossRef]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. J. Agric. Food Chem. 2005, 29, 297–303. [Google Scholar]
- Mareezek, A.; Leja, M.; Ben, J. Total phenolics, anthocyanins and antioxidant activity in the peel of the stored apples. J. Fruit Ornamental Plant Res. 2000, 8, 59–64. [Google Scholar]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondella, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Association of Official Analytical Chemists (AOAC), Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed; AOAC Inc.: Arlington, VA, USA, 1990.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their screening effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brands-William, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 307–315. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Sahito, A.; Kazi, T.G.; Jakhrani, M.A.; Kazi, G.H.; Shar, G.Q.; Memon, M.A. Elemental investigation of Momordica charantia Linn., and Syziginm jambolana Linn., using atomic absorption spectrophotometer. Nucleus 2002, 39, 49–54. [Google Scholar]
- Sample Availability: Samples of the fruits are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manzoor, M.; Anwar, F.; Saari, N.; Ashraf, M. Variations of Antioxidant Characteristics and Mineral Contents in Pulp and Peel of Different Apple (Malus domestica Borkh.) Cultivars from Pakistan. Molecules 2012, 17, 390-407. https://doi.org/10.3390/molecules17010390
Manzoor M, Anwar F, Saari N, Ashraf M. Variations of Antioxidant Characteristics and Mineral Contents in Pulp and Peel of Different Apple (Malus domestica Borkh.) Cultivars from Pakistan. Molecules. 2012; 17(1):390-407. https://doi.org/10.3390/molecules17010390
Chicago/Turabian StyleManzoor, Maleeha, Farooq Anwar, Nazamid Saari, and Muhammad Ashraf. 2012. "Variations of Antioxidant Characteristics and Mineral Contents in Pulp and Peel of Different Apple (Malus domestica Borkh.) Cultivars from Pakistan" Molecules 17, no. 1: 390-407. https://doi.org/10.3390/molecules17010390




