Schiff Bases of Indoline-2,3-dione: Potential Novel Inhibitors of Mycobacterium Tuberculosis (Mtb) DNA Gyrase †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

2.2. Mtb DNA Gyrase Inhibitory Activity

| Compd. No. | Clog P a | Mtb wt gyrase IC50 (μM) b |
|---|---|---|
| 3 | 0.72 | >200 |
| 4 | 1.4 | >200 |
| 5 | 0.87 | >200 |
| 6 | 1.22 | >200 |
| 7 | 1.75 | >200 |
| 8 | 0.47 | 157.3 |
| 9 | 2.64 | >200 |
| 10 | 3.37 | >200 |
| 11 | 4.08 | >200 |
| 12 | 3.51 | >200 |
| 13 | 3.87 | >200 |
| 14 | 0.72 | >200 |
| 15 | 1.54 | >200 |
| 16 | 1.14 | >200 |
| 17 | 3.49 | >200 (294) |
| 18 | 3.28 | ~50, 56.3 |
| 19 | 2.52 | 89.9 |
| 20 | 4.7 | >200 |
| 21 | 2.67 | >200 |
| 22 | 5.36 | >200 |
| 23 | 7.29 | >200 |
| 24 | 6.53 | >200 |
| 25 | 6.14 | >200 |
| 26 | 0.27 | >200 |
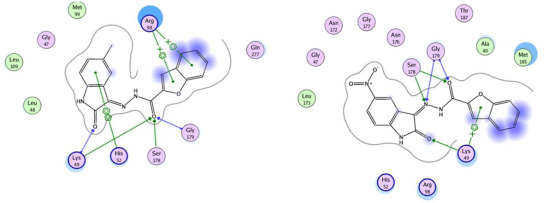
2.3. Molecular Modeling Study
| Compd. No. | No. of poses in major cluster | Binding energy (kcal/mol) | Hydrogen bond residues | Other contacts |
|---|---|---|---|---|
| 8 | 41 | −7.02 | Arg98, Asn172, Ser178 | Lys49 |
| 17 | 35 | −3.21 | Lys49, Ser178 | - |
| 18 | 63 | −8.09 | Lys49, Ser178, Gly179 | His52, Arg98 |
| 19 | 48 | −6.85 | Lys49, Ser178, Gly179 | Lys49 |


3. Conclusions
4. Experimental
4.1. General
4.2. General Procedure for the Synthesis of Schiff Bases 3-26:
4.3. Mycobacterium Tuberculosis (Mtb) Gyrase Supercoiling Assay
Acknowledgements
- Sample Availability: Samples of the compounds are available from Prof. Tarek Aboul-Fadl, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia.
References
- Chatterji, M.; Unniraman, S.; Mahadevan, S.; Nagaraja, V. Effect of different classes of inhibitors on DNA gyrase from Mycobacterium smegmatis. J. Antimicrob. Chemother. 2001, 48, 479–485. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., III.; Tekaia, F.; Badcock, K.; Basham, D.; Brown, D.; Chillingworth, T.; Connor, R.; Davies, R.; Devlin, K.; Feltwell, T.; Gentles, S.; Hamlin, N.; Holroyd, S.; Hornsby, T.; Jagels, K.; Krogh, A.; McLean, J.; Moule, S.; Murphy, L.; Oliver, K.; Osborne, J.; Quail, M.A.; Rajandream, M.-A.; Rogers, J.; Rutter, S.; Seeger, K.; Skelton, J.; Squares, R.; Squares, S.; Sulston, J.E.; Taylor, K.; Whitehead, S.; Barrell, B.G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar]
- Aubry, A.; Fisher, L.M.; Jarlier, V.; Cambau, E. First functional characterization of a singly expressed bacterial type II topoisomerase: The enzyme from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2006, 348, 158–165. [Google Scholar] [CrossRef]
- Maxwell, A. DNA gyrase as a drug target. Trends Microbiol. 1997, 5, 102–109. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Borcherding, S.M.; Stevens, R.; Nicholas, R.A.; Corley, C.R.; Self, T. Quinolones: A practical review of clinical uses, dosing considerations, and drug interaction. J. Fam. Pract. 1996, 42, 69–78. [Google Scholar]
- Brvar, M.; Perdih, A.; Oblak, M.; Mašic¡, L.P.; Solmajer, T. In silico discovery of 2-amino-4-(2,4-dihydroxyphenyl)thiazoles as novel inhibitors of DNA gyrase B. Bioorg. Med. Chem. Lett. 2010, 20, 958–962. [Google Scholar]
- Bradbury, B.J.; Pucci, M.J. Recent advances in bacterial topoisomerase inhibitors. Curr. Opp. Pharm. 2008, 8, 574–581. [Google Scholar] [CrossRef]
- Oblak, M.; Kotnik, M.; Solmajer, T. Discovery and development of ATPase inhibitors of DNA gyrase as antibacterial agents. Curr. Med. Chem. 2007, 14, 2033–2047. [Google Scholar]
- Boehm, H.J.; Boehringer, M.; Bur, D.; Gmuender, H.; Huber, W.; Klaus, W.; Kostrewa, D.; Kuehne, H.; Luebbers, T.; Meunier-Keller, N.; Mueller, F. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J. Med. Chem. 2000, 43, 2664–2674. [Google Scholar] [CrossRef]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 2004, 47, 3693–3696. [Google Scholar]
- Charifson, P.S.; Grillot, A.L.; Grossman, T.H.; Parsons, J.D.; Badia, M.; Bellon, S.; Deininger, D.D.; Drumm, J.E.; Gross, C.H.; LeTiran, A.; Liao, Y.; Mani, N.; Nicolau, D.P.; Perola, E.; Ronkin, S.; Shannon, D.; Swenson, L.L.; Tang, Q.; Tessier, P.R.; Tian, S.-K.; Trudeau, M.; Wang, T.; Wei, Y.; Zhang, H.; Stamos, D. Novel dual-targeting benzimidazole urea inhibitors of DNA gyrase and topoisomerase IV possessing potent antibacterial activity: Intelligent design and evolution through the judicious use of structure-guided design and structure-activity relationships. J. Med. Chem. 2008, 51, 5243–5263. [Google Scholar]
- Luebbers, T.; Anghern, P.; Gmuender, H.; Herzig, S. Design, synthesis, and structure-activity relationship studies of new phenolic DNA gyrase inhibitor. Bioorg. Med. Chem. Lett. 2007, 17, 4708–4714. [Google Scholar] [CrossRef]
- Oblak, M.; Golic Grdadolnik, S.; Kotnik, M.; Jerala, R.; Filipic, M.; Solmajer, T. In silico fragment-based discovery of indolin-2-one analogues as potent DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 2507–2510. [Google Scholar]
- Sriram, D.; Aubry, A.; Yogeeswari, P.; Fisher, L.M. Gatifloxacin derivatives: synthesis, antimycobacterial activities, and inhibition of Mycobacterium tuberculosis DNA gyrase. Bioorg. Med. Chem. Lett. 2006, 16, 2982–2985. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Basha, J.S.; Radha, D.R.; Nagaraja, V. Synthesis and antimycobacterial evaluation of various 7-substituted ciprofloxacin derivatives. Bioorg. Med. Chem. 2005, 13, 5774–5778. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Bin-Jubair, F.A.S. Anti-Tubercular Activity of Isatin Derivatives. Int. J. Res. Pharm. Sci. 2010, 1, 113–126. [Google Scholar]
- Aboul-Fadl, T.; Abdel-Aziz, H.A.; Kadi, A.; Bari, A.; Ahmad, P.; Al-Samani, T.; Ng, S.W. Microwave-assisted one-step synthesis of fenamic acid hydrazides from the corresponding acids. Molecules 2011, 16, 3544–3551. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Abdel-Aziz, H.A.; Kadi, A.; Ahmad, P.; Elsaman, T.; Attwa, M.W.; Darwish, I.A. Microwave-Assisted Solution-Phase Synthesis and DART-Mass Spectrometric Monitoring of Combinatorial Library of Indolin-2,3-Dione Schiff Bases with Potential Antimycobacterial Activity. Molecules 2011, 16, 5194–5206, and the references cited therein. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Madhusudan, K.; Visweswariah, S.S.; Nagaraja, V. Structural heterogeneity in DNA gyrases from Gram-positive and Gram-negative bacteria. Curr. Sci. 2000, 79, 968–974. [Google Scholar]
- Molecular Operating Environment (MOE 2005.06) version 2008.02; C.C.G.I.: Montreal, Quebec, Canada.
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791, Autodock Version 4.2.3. The Scripps Research Institute. [Google Scholar] [CrossRef]
- Tretter, E.M.; Schoeffler, A.J.; Weisfield, S.R.; Berger, J.M. Crystal structure of the DNA gyrase GyrA N-terminal domain from Mycobacterium tuberculosis. Proteins 2010, 78, 492–495. [Google Scholar] [CrossRef]
- Ghosh, P.; Bagchi, M.C. Anti-tubercular drug designing by structure based screening of combinatorial libraries. J. Mol. Model. 2011, 17, 1607–1620. [Google Scholar] [CrossRef]
- Leo, A.J. Calculating log Poct from structures. Chem. Rev. 1993, 93, 1281–1306. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aboul-Fadl, T.; Abdel-Aziz, H.A.; Abdel-Hamid, M.K.; Elsaman, T.; Thanassi, J.; Pucci, M.J. Schiff Bases of Indoline-2,3-dione: Potential Novel Inhibitors of Mycobacterium Tuberculosis (Mtb) DNA Gyrase. Molecules 2011, 16, 7864-7879. https://doi.org/10.3390/molecules16097864
Aboul-Fadl T, Abdel-Aziz HA, Abdel-Hamid MK, Elsaman T, Thanassi J, Pucci MJ. Schiff Bases of Indoline-2,3-dione: Potential Novel Inhibitors of Mycobacterium Tuberculosis (Mtb) DNA Gyrase. Molecules. 2011; 16(9):7864-7879. https://doi.org/10.3390/molecules16097864
Chicago/Turabian StyleAboul-Fadl, Tarek, Hatem A. Abdel-Aziz, Mohammed K. Abdel-Hamid, Tilal Elsaman, Jane Thanassi, and Michael J. Pucci. 2011. "Schiff Bases of Indoline-2,3-dione: Potential Novel Inhibitors of Mycobacterium Tuberculosis (Mtb) DNA Gyrase" Molecules 16, no. 9: 7864-7879. https://doi.org/10.3390/molecules16097864
APA StyleAboul-Fadl, T., Abdel-Aziz, H. A., Abdel-Hamid, M. K., Elsaman, T., Thanassi, J., & Pucci, M. J. (2011). Schiff Bases of Indoline-2,3-dione: Potential Novel Inhibitors of Mycobacterium Tuberculosis (Mtb) DNA Gyrase. Molecules, 16(9), 7864-7879. https://doi.org/10.3390/molecules16097864