Stopped-Flow Spectrophotometric Study of the Kinetics and Mechanism of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Cation and the Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]− Anion in Aqueous Solution
Abstract
:1. Introduction
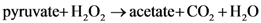
2. Results and Discussion
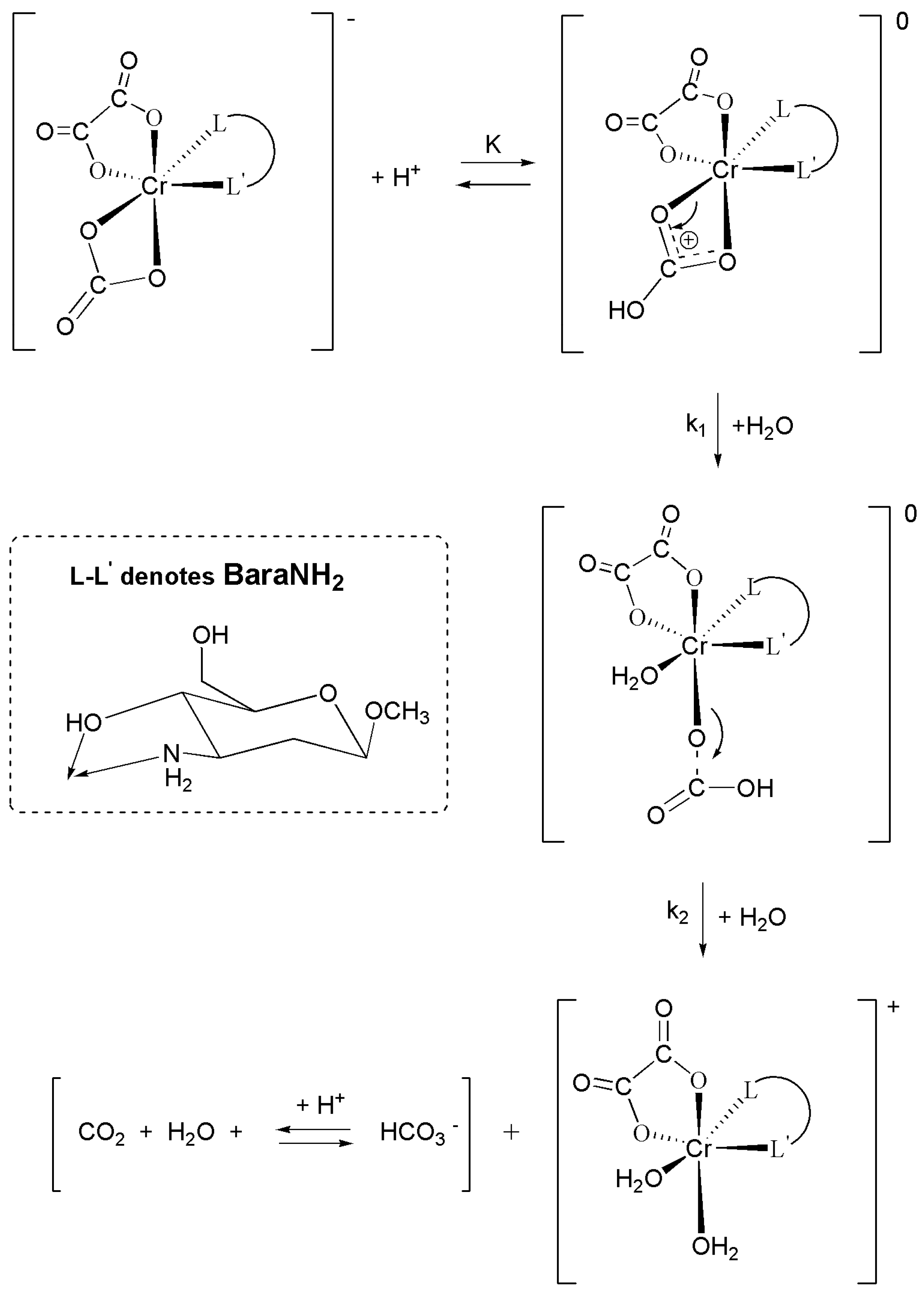
2.1. Kinetics and Mechanisms of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Complex Cation
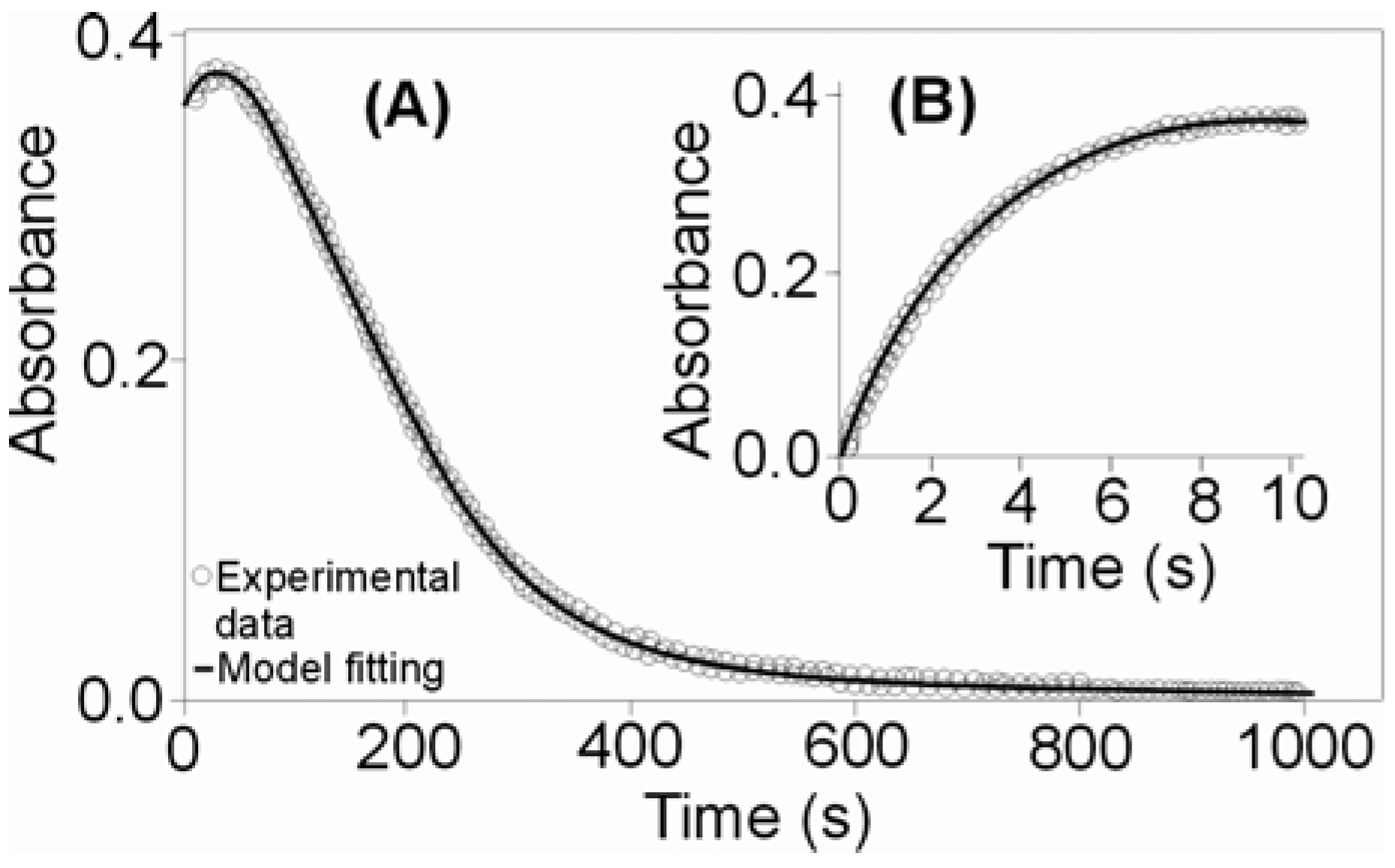
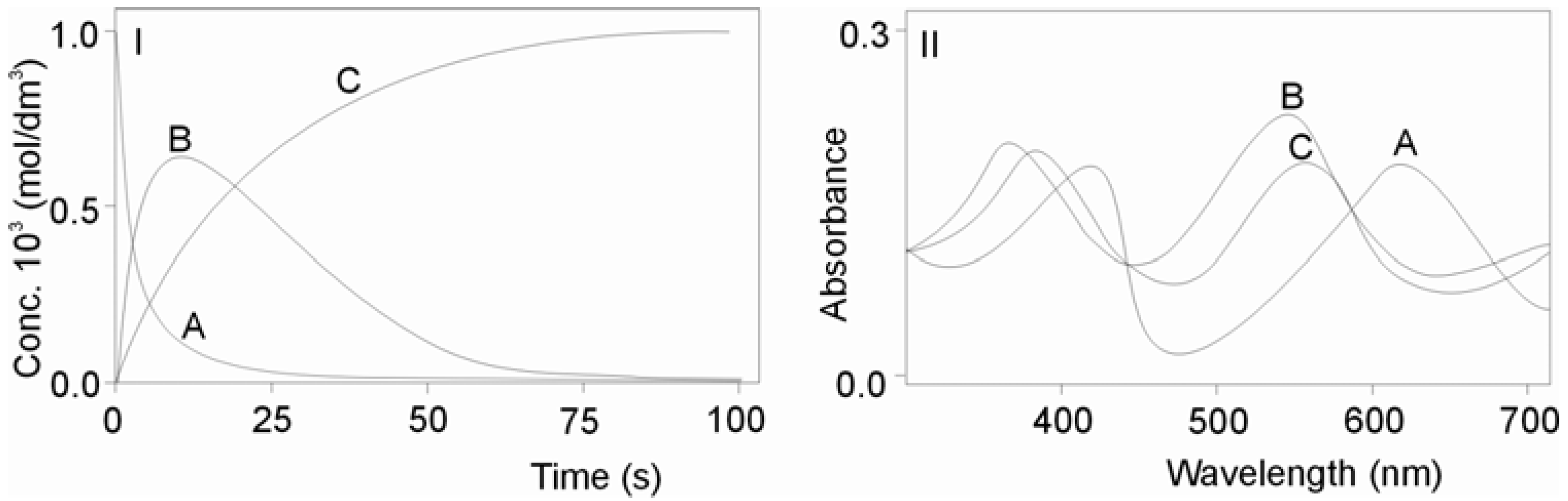
2.1.1. First Step for the Reaction of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Complex Cation
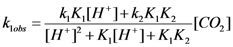
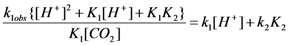
| pH | k1obs [s−1] T = 278 K | k1obs [s−1] T = 283 K | k1obs [s−1] T = 288 K | k1obs [s−1] T = 293 K | k1obs [s−1] T = 298 K |
|---|---|---|---|---|---|
| 6.82 | 0.12 ± 5.4E-3 | 0.14 ± 2.7E-3 | 0.15 ± 8.4E-3 | 0.17 ± 5.4E-3 | 0.19 ± 4.7E-3 |
| 7.12 | 0.12 ± 3.7E-3 | 0.15 ± 6.4E-3 | 0.16 ± 9.4E-3 | 0.18 ± 2.6E-3 | 0.20 ± 1.8E-3 |
| 7.45 | 0.13 ± 6.4E-3 | 0.15 ± 3.7E-3 | 0.16 ± 3.7E-3 | 0.18 ± 4.7E-3 | 0.21 ± 9.5E-3 |
| 7.89 | 0.13 ± 2.7E-3 | 0.16 ± 8.4E-3 | 0.17 ± 9.4E-3 | 0.19 ± 8.4E-3 | 0.22 ± 6.8E-3 |
| 8.22 | 0.15 ± 8.4E-3 | 0.17 ± 9.3E-3 | 0.18 ± 3.7E-3 | 0.20 ± 4.7E-3 | 0.24 ± 8.4E-3 |
| 8.54 | 0.16 ± 3.8E-3 | 0.18 ± 2.7E-3 | 0.18 ± 3.7E-3 | 0.21 ± 2.8E-3 | 0.24 ± 9.3E-3 |
| 8.92 | 0.17 ± 9.4E-3 | 0.18 ± 7.4E-3 | 0.19 ± 8.4E-3 | 0.22 ± 8.4E-3 | 0.25 ± 6.9E-3 |
| T[K] | k1[s−1M−1] (pK1) | k2[s−1M−1] (pK2) |
|---|---|---|
| 278 | 24.73 ± 0.8 (6.82 ± 0.01) | 16.28 ± 0.4 (8.91 ± 0.02) |
| 283 | 32.40 ± 0.7 (6.81 ± 0.02) | 25.45 ± 0.9 (8.91 ± 0.03) |
| 288 | 40.29 ± 1.1 (6.80 ± 0.01) | 34.34 ± 0.8 (8.90 ± 0.02) |
| 293 | 48.03 ± 1.3 (6.80 ± 0.01) | 42.29 ± 1.2 (8.91 ± 0.03) |
| 298 | 56.20 ± 1.0 (6.80 ± 0.01) | 49.07 ± 1.1 (8.90 ± 0.03) |
| ΔH# | 23.78 ± 0.87 | 16.19 ± 0.45 |
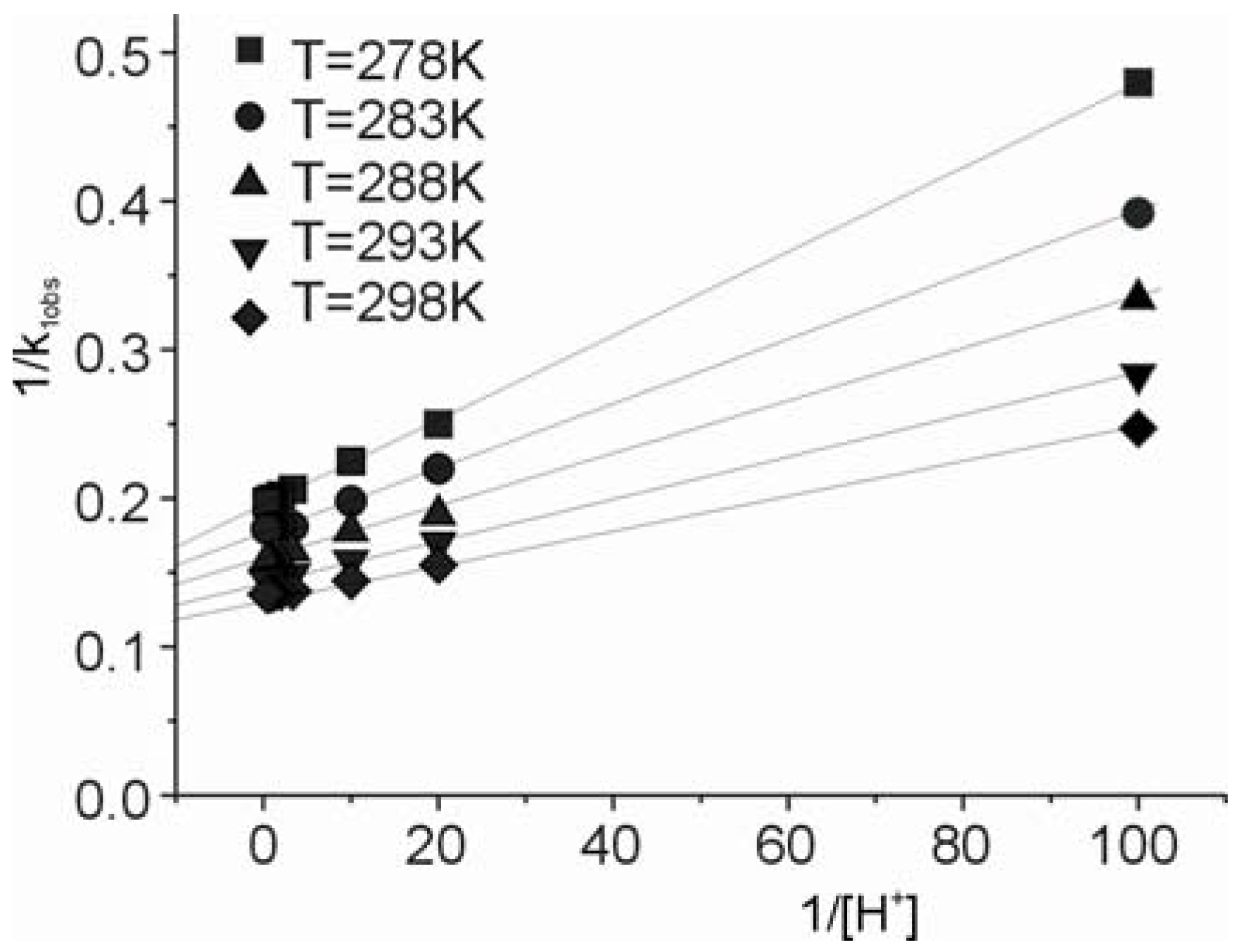
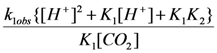 versus [H+] for the reaction of carbon dioxide uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ complex cation.
versus [H+] for the reaction of carbon dioxide uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ complex cation.
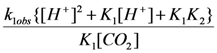 versus [H+] for the reaction of carbon dioxide uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ complex cation.
versus [H+] for the reaction of carbon dioxide uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ complex cation.
2.1.2. Second Step—The Carbonate Ring Closure
| pH | k2obs [s−1] T = 278 K | k2obs [s−1] T = 283 K | k2obs [s−1] T = 288 K | k2obs [s−1] T = 293 K | k2obs [s−1] T = 298 K |
|---|---|---|---|---|---|
| 6.82 | 1.61E-3 ± 6.4E-5 | 1.86E-3 ± 7.5E-5 | 2.35E-3 ± 4.3E-5 | 3.11E-3 ± 8.5E-5 | 4.78E-3 ± 8.4E-5 |
| 7.12 | 1.36E-3 ± 6.4E-5 | 1.58E-3 ± 8.4E-5 | 1.93E-3 ± 2.5E-5 | 2.39E-3 ± 3.5E-5 | 3.32E-3 ± 3.6E-5 |
| 7.45 | 1.19E-3 ± 7.3E-5 | 1.35E-3 ± 4.6E-5 | 1.59E-3 ± 7.4E-5 | 1.93E-3 ± 6.4E-5 | 2.46E-3 ± 2.6E-5 |
| 7.89 | 1.06E-3 ± 8.4E-5 | 1.24E-3 ± 2.5E-5 | 1.39E-3 ± 8.4E-5 | 1.64E-3 ± 2.5E-5 | 2.01E-3 ± 1.8E-5 |
| 8.22 | 9.81E-4 ± 3.6E-6 | 1.08E-3 ± 5.3E-5 | 1.22E-3 ± 4.3E-5 | 1.41E-3 ± 7.5E-5 | 1.67E-3 ± 3.4E-5 |
| 8.54 | 1.04E-4 ± 2.8E-6 | 1.19E-3 ± 8.4E-5 | 1.37E-3 ± 2.6E-5 | 1.61E-3 ± 3.5E-5 | 1.98E-3 ± 2.7E-5 |
| 8.92 | 8.06E-4 ± 7.4E-6 | 8.83E-4 ± 1.7E-6 | 9.68E-4 ± 8.4E-6 | 1.08E-3 ± 5.6E-5 | 1.24E-3 ± 8.4E-5 |
2.1.3. Proposed Mechanism of Carbon Dioxide Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Complex Cation
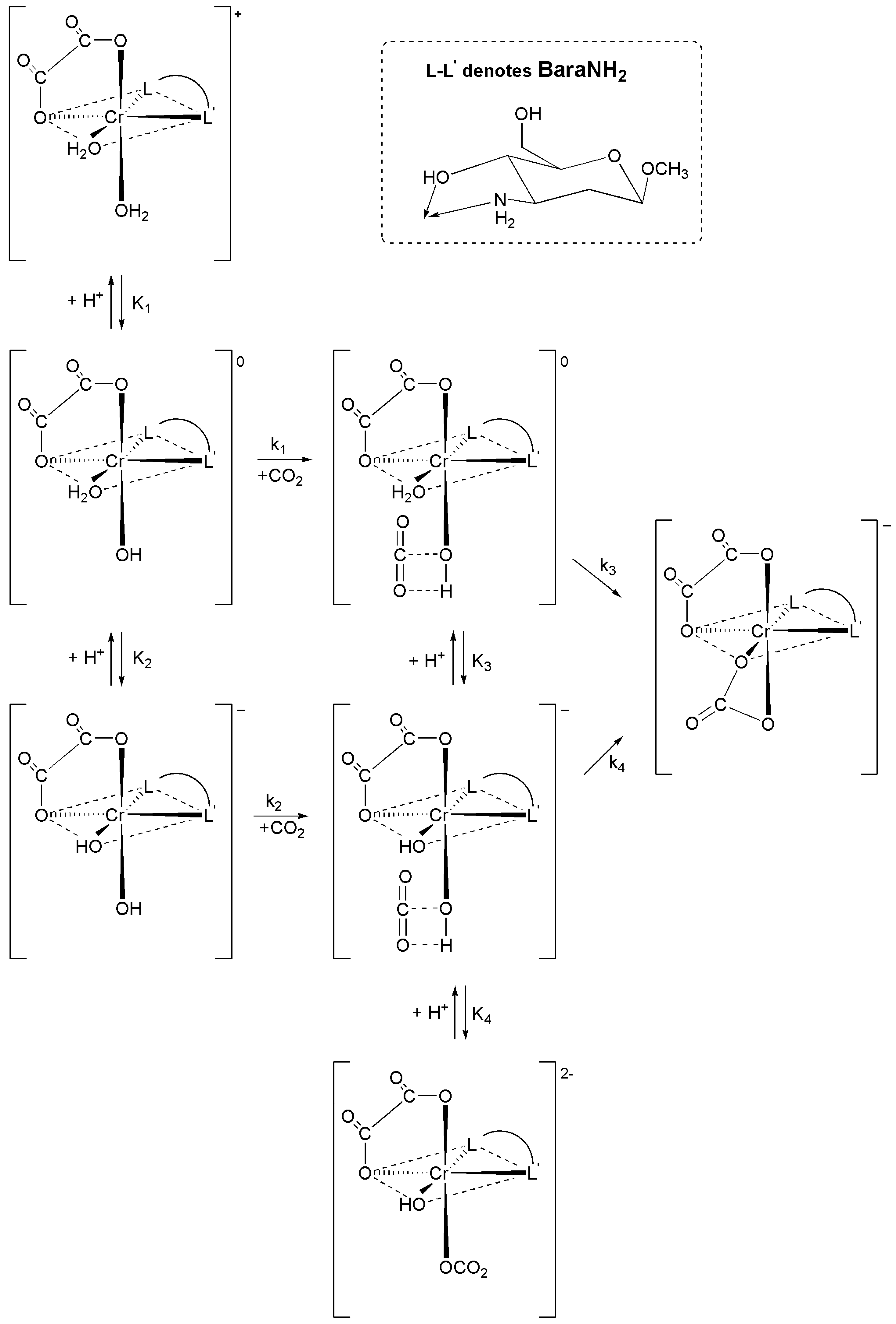
2.2. Acid Hydrolysis of the cis-[Cr(C2O4)(BaraNH2)OCO2]− Complex Anion
| k1obs [s−1] | |||||
|---|---|---|---|---|---|
| [cHCLO4] | T = 278 K | T = 283 K | T = 288 K | T = 293 K | T = 298 K |
| 0.01 | 2.08 ± 0.02 | 2.53 ± 0.08 | 2.99 ± 0.02 | 3.53 ± 0.05 | 4.05 ± 0.04 |
| 0.05 | 4.00 ± 0.03 | 4.54 ± 0.05 | 5.29 ± 0.05 | 6.33 ± 0.04 | 6.45 ± 0.06 |
| 0.1 | 4.86 ± 0.06 | 5.05 ± 0.09 | 5.62 ± 0.04 | 6.71 ± 0.09 | 6.92 ± 0.02 |
| 0.3 | 4.95 ± 0.08 | 5.51 ± 0.08 | 5.82 ± 0.08 | 6.77 ± 0.02 | 7.27 ± 0.05 |
| 0.5 | 5.00 ± 0.02 | 5.52 ± 0.09 | 6.34 ± 0.07 | 6.83 ± 0.05 | 7.32 ± 0.03 |
| 0.8 | 5.02 ± 0.06 | 5.53 ± 0.04 | 6.35 ± 0.03 | 6.84 ± 0.06 | 7.35 ± 0.05 |
| 1.1 | 5.03 ± 0.04 | 5.54 ± 0.06 | 6.36 ± 0.06 | 6.85 ± 0.02 | 7.36 ± 0.02 |
| 1.4 | 5.03 ± 0.09 | 5.54 ± 0.03 | 6.37 ± 0.02 | 6.86 ± 0.08 | 7.37 ± 0.03 |
| 1.7 | 5.04 ± 0.01 | 5.55 ± 0.02 | 6.38 ± 0.08 | 6.87 ± 0.04 | 7.38 ± 0.04 |
| 2.0 | 5.05 ± 0.03 | 5.56 ± 0.05 | 6.39 ± 0.03 | 6.88 ± 0.02 | 7.39 ± 0.07 |
| 2.3 | 5.05 ± 0.02 | 5.57 ± 0.07 | 6.40 ± 0.08 | 6.89 ± 0.02 | 7.39 ± 0.05 |
| 2.7 | 5.06 ± 0.05 | 5.58 ± 0.01 | 6.42 ± 0.02 | 6.89 ± 0.06 | 7.39 ± 0.06 |
| T[K] | k1 [s−1] | k2 [s−1] = k2obs | K [M−1] |
|---|---|---|---|
| 278 | 5.10 ± 0.09 | 5.42 ± 0.09 | 1.14 ± 0.02 |
| 283 | 5.62 ± 0.08 | 6.47 ± 0.08 | 1.15 ± 0.01 |
| 288 | 6.29 ± 0.09 | 7.52 ± 0.08 | 1.15 ± 0.02 |
| 293 | 6.92 ± 0.08 | 8.47 ± 0.07 | 1.14 ± 0.02 |
| 298 | 7.43 ± 0.09 | 9.53 ± 0.09 | 1.14 ± 0.03 |
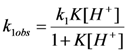
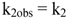
2.2.1. Proposed Mechanism for the Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]− Complex Anion in Aqueous Solution
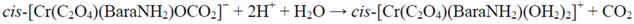
3. Experimental
3.1. Reagents
3.2. Synthesis of Methyl 3-amino-2,3-dideoxy-β-D-arabinohexopyranoside (BaraNH2)
3.3. Synthesis of cis-[Cr(C2O4)(BaraNH2)(OH2)2]+
3.4. Synthesis of the cis-[Cr(C2O4)(BaraNH2)OCO2]−
3.5. Spectral Measurements
3.6. Determination of Acidity Constants of cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Complex Ion

3.7. Determination of Acidity Constant for the cis-[Cr(C2O4)(BaraNH2)OCO2]- Complex Ion
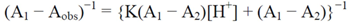
3.8. Kinetic Measurements for the Reaction of CO2 Uptake by cis-[Cr(C2O4) (BaraNH2)(OH2)2]+
3.9. Kinetic Measurements for the Reaction of Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]−
4. Conclusions
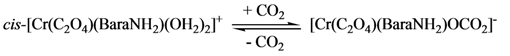
Acknowledgements
References
- Maccoll, P.; Carlos, R. Carbonato complexes of cobalt(III). Coord. Chem. Rev. 1969, 2, 147–198. [Google Scholar]
- Harris, G.M.; Krishnamurthy, K.V.; Sastri, V.S. Chemistry of the metal carbonato complexes. Chem. Rev. 1970, 70, 171–197. [Google Scholar]
- Min, D.; Lee, S.W. Terbium-oxalate-pyridinedicarboxylate coordination polymers suggesting the reductive coupling of carbon dioxide (CO2) to oxalate (C2O42−):[Tb2(3,5-PDC)2(H2O)4(C2O4)]·2H2O and [Tb(2,4-PDC)(H2O)(C2O4)0.5] (PDC = pyridinedicarboxylate). Inorg. Chem. Commun. 2002, 5, 978–983. [Google Scholar] [CrossRef]
- Fujita, E. Photochemical carbon dioxide reduction with metal complexes. Coord. Chem. Rev. 1999, 185-186, 373–384. [Google Scholar]
- Yin, X.; Moss, J.R. Recent developments in the activation of carbon dioxide by metal complexes. 1999, 181, 27–59. [Google Scholar]
- Ni, J.; Qiu, Y.; Cox, T.M.; Jones, C.A.; Berry, C.; Melon, L.; Bott, S. Carbon dioxide chemistry: Characterization of the carbon dioxide reaction product of a dinuclear titanium complex. Organomet 1996, 15, 4669–4671. [Google Scholar] [CrossRef]
- Hanna, T.A.; Baranger, A.M.; Bergman, R.G. Reaction of carbon dioxide and heterocumulenes with an unsymmetrical metal-metal bond. Direct addition of carbon dioxide across a zirconium-iridium bond and stoichiometric reduction of carbon dioxide to formate. J. Am. Chem. Soc. 1995, 117, 11363–11364. [Google Scholar]
- Antiñolo, A.; Fajardo, M.; García-Yuste, S.; del Hierro, I.; Otero, A.; Elkrami, S.; Mourad, Y.; Mugnier, Y. Synthesis, electrochemistry and reactivity of formato- and acetate-niobocene complexes. J. Chem. Soc. Dalton Trans. 1995, 3409–3414. [Google Scholar]
- Saotome, C.; Ono, M.; Akita, H. Chemoenzymatic syntheses of N-trifluoroacetyl-L-daunosamine, N-trifluoroacetyl-L-acosamine, N-benzoyl-D-acosamine and N-benzoyl-D-ristosamine from an achiral precursor, methyl sorbate. Tetrahedron: Asymmetry 2000, 11, 4137–4151. [Google Scholar] [CrossRef]
- Kozłowski, H.; Decock, P.; Olivier, I.; Micera, G.; Pusino, A.; Pettit, L.D. Stability and structure of copper(II) complexes with 2-amino-2-deoxy-D-mannose and some derivatives. Carbohydr. Res. 1990, 197, 109–117. [Google Scholar] [CrossRef]
- Jeżowska-Bojczuk, M.; Kozłowski, H.; Decock, P.; Cerny, M.; Trnka, T. Potentiometric and spectroscopic studies of the binding of copper(II) ions by aminodeoxy derivatives of 1,6-anhydro-β-D-glucopyranose. Carbohydr. Res. 1992, 216, 453–460. [Google Scholar] [CrossRef]
- Jeżowska-Bojczuk, M.; Kozłowski, H.; Trnka, T.; Cerny, M. Interaction of 1,6-anhydro derivatives of amino sugars with copper(II) ions. Carbohydr. Res. 1994, 253, 19–28. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Sikorski, A.; Jacewicz, D.; Chmurzyński, L. X-ray and conformational analysis of methyl 3-amino-2,3-dideoxy-α-D-arabino-hexopyranoside. Carbohydr. Res. 2004, 339, 1195–1199. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Sikorski, A.; Jacewicz, D.; Chmurzyński, L. Crystal structure of methyl 3-amino-2,3-dideoxy-β-D-arabino-hexopyranoside. Stabilization of the crystal lattice by a double network of N-H…O (O-H…N) and C-H…O interactions. Carbohydr. Res. 2005, 340, 2001–2005. [Google Scholar]
- Dąbrowska, A.; Jacewicz, D.; Makowska, J.; Makowski, M.; Chmurzyński, L. Ab initio study of the energetics of protonation and deprotonation of the methyl 3-amino-2,3-dideoxyhexopyranosides isomers. J. Mol. Struct. Theochem 2005, 718, 87–92. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Jacewicz, D.; Łapińska, A.; Banecki, B.; Figarski, A.; Szkatuła, M.; Lehman, J.; Krajewski, J.; Kubasik-Juraniec, J.; Woźniak, M.; et al. Pivotal participation of nitrogen dioxide in L-arginine induced acute necrotizing pancreatitis; protective role of superoxide scavenger 4-OH TEMPO. Biochem. Biophys. Res. Commun. 2005, 326, 313–320. [Google Scholar] [CrossRef]
- Jacewicz, D.; Dabrowska, A.; Wyrzykowski, D.; Pranczk, J.; Wozniak, M.; Kubasik-Juraniec, J.; Knap, N.; Siedlecka, K.; Neuwelt, A.J.; Chmurzynski, L. A novel biosensor for evaluation of apoptotic or necrotic effects of nitrogen dioxide during acute pancreatitis in rat. Sensors 2010, 10, 280–229. [Google Scholar]
- Cavallini, L.; Valente, M.; Rigobello, M.P. The protective action of pyruvate on recovery of ischemic rat heart: Comparison with other oxidizable substrates. J. Mol. Cell. Cardiol. 1990, 22, 143–154. [Google Scholar] [CrossRef]
- Bunger, R.; Swindall, B.; Brodie, D.; Zdunek, D.; Stiegler, H.; Walter, G. Pyruvate attenuation of hypoxia damage in isolated working guinea-pig hart. J. Mol. Cell. Cardiol. 1986, 18, 423–438. [Google Scholar]
- Mentzer, R.M., Jr.; Van Wylen, D.G.L.; Shodi, J. Effect of pyruvate on regional ventricular function in normal and stunned myocardium. Ann. Surg. 1989, 209, 629–634. [Google Scholar] [CrossRef]
- Helferich, F.G. Kinetics of Homogeneous Multistep Reactions; Elsevier: New York, NY, USA, 2001; pp. 11–16. [Google Scholar]
- Chaffee, E.; Dasgupta, T.P.; Harris, G.M. Kinetics and mechanism of aquation and formation reactions of carbonato complexes. V. Carbon dioxide uptake by hydroxopentaamminecobalt(III) ion to form carbonatopentaamminecobalt(III) ion. J. Am. Chem. Soc. 1973, 95, 4169–4173. [Google Scholar] [CrossRef]
- Dasgupta, T.P.; Harris, G.M. Kinetics and mechanism of aquation and formation reactions of carbonato complexes. 11. Carbon dioxide uptake and intramolecular carbonato ligand chelation in aqueous solution of cis- and trans-diaquo(1,4,8,11-tetraazacyclotetradecane)cobalt(III) cations. J. Am. Chem. Soc. 1977, 99, 2490–2498. [Google Scholar] [CrossRef]
- Dasgupta, T.P.; Harris, G.M. Kinetics and mechanism of aquation and formation reactions of carbonato complexes. VII. Acid-catalyzed aquation of carbonato(nitrilotriacetato)cobaltate(III) ion. Inorg. Chem. 1974, 13, 1275–1278. [Google Scholar] [CrossRef]
- van Eldik, R.; Dasgupta, T.P.; Harris, G.M. Kinetics and mechanism of aquation and formation reactions of carbonato complexes. IX. Aquation of α- and β-cis carbonato(ethylenediaminediacetato)cobaltate(III) ions in strongly acidic solution. Inorg. Chem. 1975, 14, 2573–2575. [Google Scholar] [CrossRef]
- Jacewicz, D.; Banecki, B.; Dąbrowska, A.; Woźniak, M.; Chmurzyński, L. Kinetics and mechanisms of the CO2 and SO2 uptake by coordinate ion, cis-[Cr(C2O4)(L-L)(OH2)2]+ {(L-L) = methyl 3-amino-2,3-dideoxy-α-D-arabino-hexopyranoside} studied by stopped-flow spectrophotometry. Inorg. Chim. Acta 2004, 357, 4467–4475. [Google Scholar] [CrossRef]
- Palmer, D.A.; Dasgupta, T.P.; Kelm, P. Kinetics and mechanism of aquation and formation reactions of cis-carbonatobis(oxalato)chromate(III) ion in aqueous solution. Inorg. Chem. 1978, 17, 1173–1176. [Google Scholar] [CrossRef]
- Buckingham, D.A.; Clark, C.R. Acid-catalyzed hydrolysis of the carbonatobis(ethylenediamine)cobalt(1+) ion revisited. Inorg. Chem. 1993, 32, 5405–5407. [Google Scholar] [CrossRef]
- Palmer, D.A.; Dasgupta, T.P.; Kelm, H. Kinetics and mechanism of aquation and formation reactions of cis-carbonatobis(oxalato)chromate(III) ion in aqueous solution. Inorg. Chem. 1978, 17, 1173–1176. [Google Scholar] [CrossRef]
- Jacewicz, D.; Łapińska, A.; Dąbrowska, A.; Chmurzyński, L. A Stopped-flow study on the kinetics and mechanizm of CO2 uptake by the cis-[Cr(1,10-phenantroline)2(OH2)2]3+ complex ion. Trans. Met. Chem. 2006, 31, 111–117. [Google Scholar] [CrossRef]
- Johanson, M.L.; Correira, J.J.; Yphantis, D.A.; Halvorson, H.R. Analysis of data from the analytical ultracentrifuge by nonlinear least-squares techniques. Biophys. J. 1981, 36, 575–588. [Google Scholar] [CrossRef]
- Nagle, J.F.; Parodi, L.A.; Lozier, R.H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys. J. 1982, 38, 161–174. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jacewicz, D.; Dąbrowska, A.; Chmurzyński, L. Stopped-Flow Spectrophotometric Study of the Kinetics and Mechanism of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Cation and the Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]− Anion in Aqueous Solution. Molecules 2011, 16, 7746-7761. https://doi.org/10.3390/molecules16097746
Jacewicz D, Dąbrowska A, Chmurzyński L. Stopped-Flow Spectrophotometric Study of the Kinetics and Mechanism of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Cation and the Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]− Anion in Aqueous Solution. Molecules. 2011; 16(9):7746-7761. https://doi.org/10.3390/molecules16097746
Chicago/Turabian StyleJacewicz, Dagmara, Aleksandra Dąbrowska, and Lech Chmurzyński. 2011. "Stopped-Flow Spectrophotometric Study of the Kinetics and Mechanism of CO2 Uptake by cis-[Cr(C2O4)(BaraNH2)(OH2)2]+ Cation and the Acid-Catalyzed Decomposition of cis-[Cr(C2O4)(BaraNH2)OCO2]− Anion in Aqueous Solution" Molecules 16, no. 9: 7746-7761. https://doi.org/10.3390/molecules16097746




