Chirality and Numbering of Substituted Tropane Alkaloids
Abstract
:1. Introduction
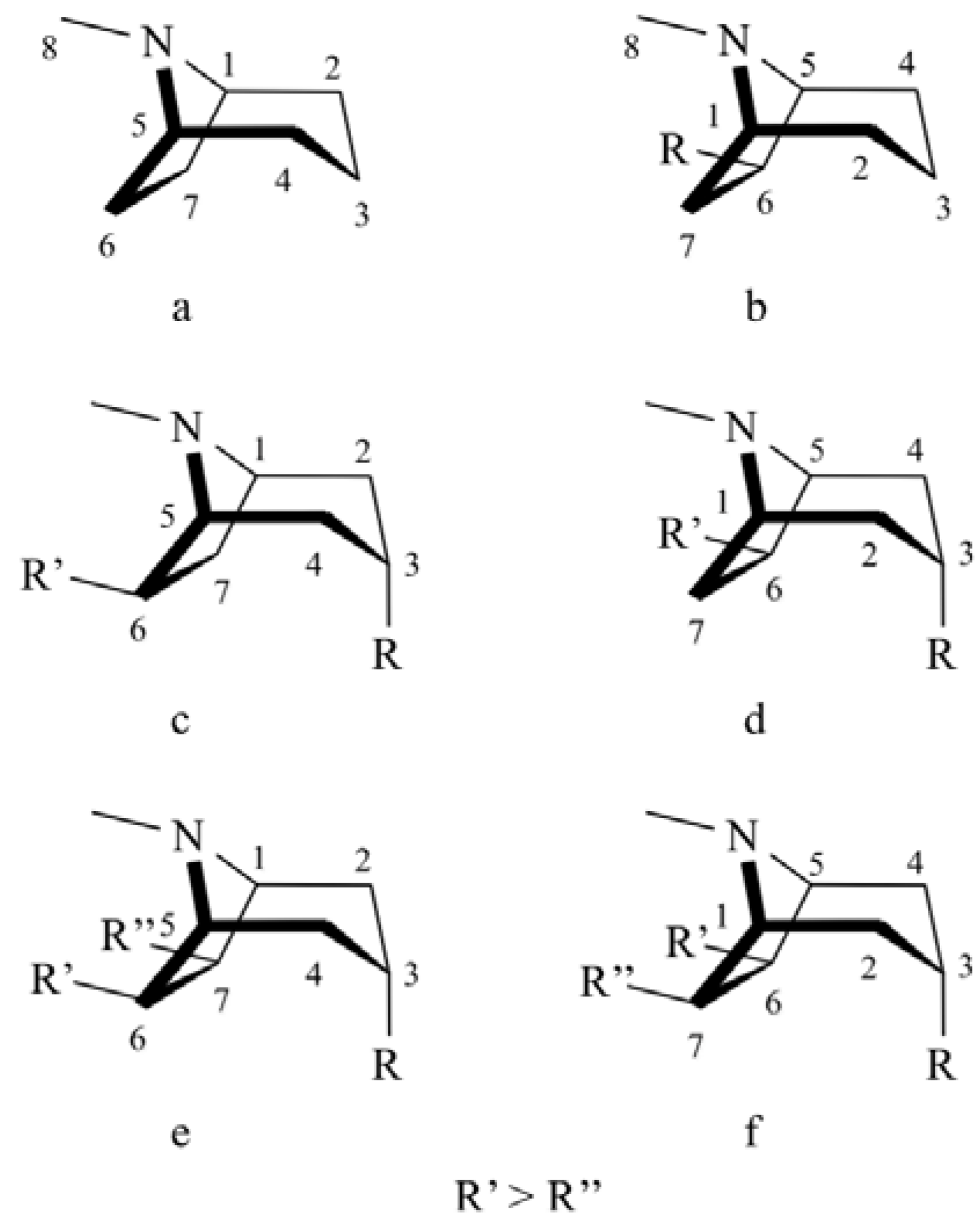
2. Determination of the Configuration Using Optical Properties
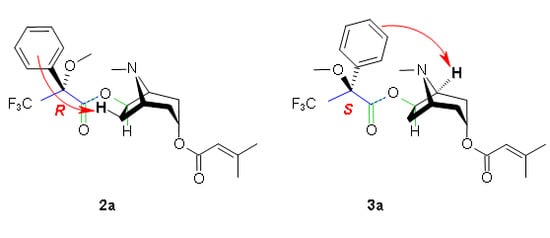
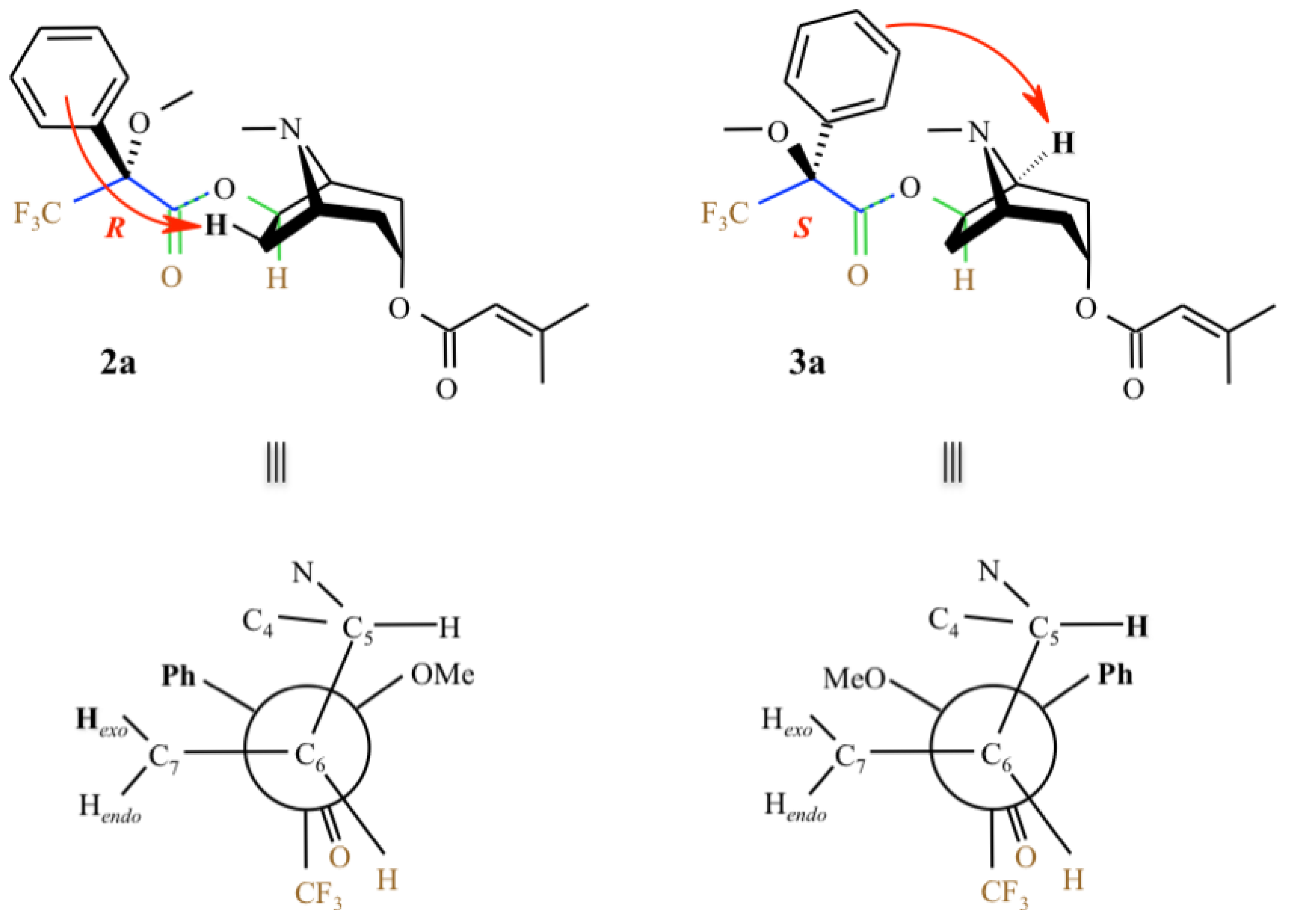
3. Determination of the Configuration Using Mosher’s Reagent
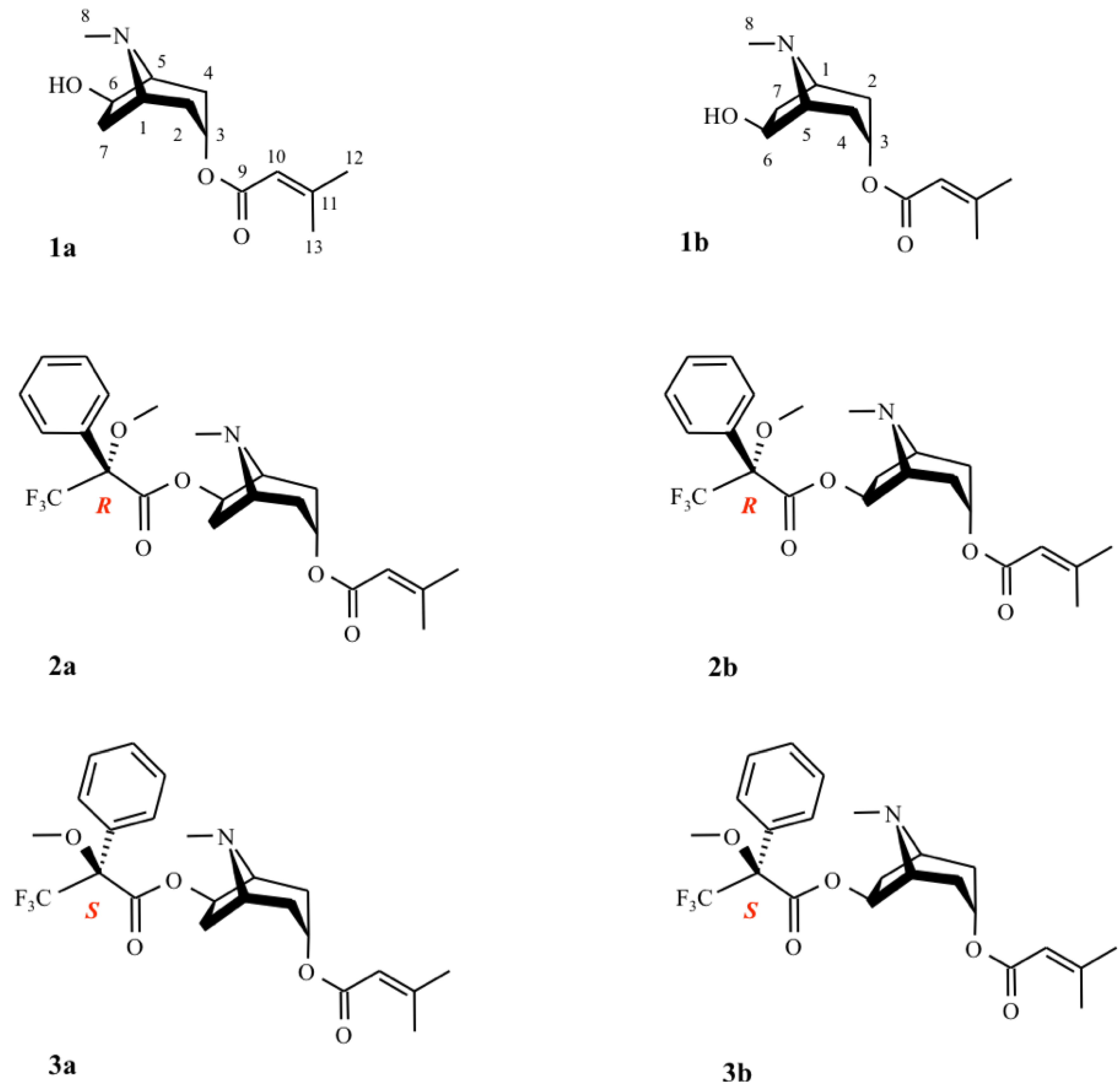
| 1a | 2a ((R)-MTPA ester) | 3a ((S)-MTPA ester) | ||||
|---|---|---|---|---|---|---|
| d (H) | d (C) | d (H) | d (C) | d (H) | d (C) | |
| H–1 | 3.79 (s) | 61.0 | 3.32 (br s) | 60.4 | 3.31 (br s) | 60.8 |
| H endo–2 | 2.56-2.45 (m) | 32.1 | 2.14-2.13 (m) | 35.3 | 2.14-2.13 (m) | 35.8 |
| H exo–2 | 1.88 (d) (J2exo,2endo = 15) | 1.71-1.67 (m) | 1.72-1.69 (m) | |||
| H–3 | 5.05 (s) | 63.0 | 5.05 (t) (J = 4.85) | 65.5 | 5.05 (t) (J = 4.96) | 65.5 |
| H endo–4 | 2.56-2.45 (m) | 30.6 | 2.20-2.19 (m) | 34.0 | 2.21-2.20 (m) | 34.4 |
| H exo–4 | 2.03 (d) (J4exo,4endo = 15) | 1.93-1.91 (m) | 1.97-1.95 (m) | |||
| H–5 | 3.67 (s) | 68.9 | 3.24 (s) | 66.1 | 3.14 (s) | 66.4 |
| H–6 | 4.80 (dd) (J6,7exo = 5) | 72.6 | 5.73 (dd) (J6,7endo = 7.48, J6,7exo = 2.97) | 82.0 | 5.76 (dd) (J6,7endo = 7.57, J6,7exo = 2.95) | 82.0 |
| H endo–7 | 2.85 (dd) (J7endo,7exo = 10, J7endo,6 = 5) | 37.5 | 2.65 (dd) (J7endo,7exo = 14, J7endo,6 = 7.5) | 35.5 | 2.63 (dd,) (J7endo,7exo = 14, J7endo,6 = 7.9) | 34.8 |
| H exo–7 | 2.30-2.25 (m) | 2.12-2.09 (m) | 2.24 (br s) | |||
| H3C–N | 2.90 (s) | 37.5 | 2.34 (s) | 40.5 | 2.24 (s) | 40.8 |
| H–10 | 5.65 (s) | 115.6 | 5.71 (t) | 116.1 | 5.70 (s) | 116.0 |
| (4J = 1.29) | ||||||
| H–12 | 2.19 (s) | 20.3 | 2.20 (s) | 20.3 | 2.20 (s) | 20.3 |
| H–13 | 1.93 (s) | 27.4 | 1.94 (s) | 27.5 | 1.94 (s) | 27.4 |
| H3C–O | 3.56 (s) | 55.4 | 3.58 (s) | 55.4 | ||
| H ortho–Ph | 7.55-7.53 (m) | 127.4 | 7.55-7.53 (m) | 127.2 | ||
| H meta–Ph | 7.43-7.41 (m) | 128.5 | 7.43-7.41 (m) | 128.4 | ||
| H para–Ph | 7.43-7.41 (m) | 129.7 | 7.43-7.41 (m) | 129.6 | ||
4. Experimental
4.1. Plant Material
4.2. Chemical and Reagents
4.3. Apparatus
4.4. Preparation of Mosher’s Esters
5. Conclusions
Acknowledgements
References
- Dominguez, B.E.; Garcia, P.E.; Cardenas, J. Determining the absolute configuration of secondary alcohols by means of a chiral auxiliary and NOESY. Tetrahedron Asymmetry 2005, 16, 3976–3985. [Google Scholar] [CrossRef]
- Wright, A.D.; Koenig, G.M.; Sticher, O. The enhanced role of a lanthanide shift reagent in the structure elucidation and proton NMR and carbon-13 NMR characterization of some marine natural products. Phytochem. Anal. 1992, 3, 73–79. [Google Scholar] [CrossRef]
- Luy, B. Disinction of enantiomers by NMR spectroscopy using chiral orienting media. J. Indian Inst. Sci. 2010, 90, 119–132. [Google Scholar]
- Berova, N.; Ellestad, G.A.; Harada, N. Modern Methods in Natural Product Chemistry: Characterization by Circular Dichroism Spectroscopy. In Comprehensive Natural Products II; Mander, L., Lui, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 9, pp. 91–147. [Google Scholar]
- Seco, J.M.; Quinoa, E.; Riguera, R. The assignment of absolute configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar]
- Kang, C.-Q.; Guo, H.-Q.; Qiu, X.-P.; Bai, X.-L.; Yao, H.-B.; Gao, L.-X. Assignment of absolute configuration of cyclic secondary amines by NMR techniques using Mosher's method: A general procedure exemplified with (-)-isoanabasine. Magn. Reson. Chem. 2006, 44, 20–24. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fujisawa, H.; Noyori, R. A Very Reliable Method for Determination of Absolute Configuration of Chiral Secondary Alcohols by 1H NMR Spectroscopy. Org. Lett. 2004, 6, 4607–4610. [Google Scholar] [CrossRef]
- Harada, N. Chiral auxiliaries powerful for both enantiomer resolution and determination of absolute configuration by X-ray crystallography. Top. Stereochem. 2006, 25, 177–203. [Google Scholar]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids. In Alkaloids; Academic Press: San Diego, CA, USA, 1993; Volume 44, pp. 1–114. [Google Scholar]
- Moss, G.P. International Union of Pure and Applied Chemistry. Commission on Nomenclature of Organic Chemistry. Available online: http://www.chem.qmul.ac.uk/iupac/sectionF/ (accessed on 24 April 2011).
- Christen, P. Tropane alkaloids: Old drugs used in modern medicine. Stud. Nat. Prod. Chem. 2000, 22, 717–749, Bioactive Natural Products (Part C). [Google Scholar] [CrossRef]
- Doncheva, T.; Berkov, S.; Philipov, S. Comparative study of the alkaloids in tribe Datureae and their chemosystematic significance. Biochem. Syst. Ecol. 2006, 34, 478–488. [Google Scholar] [CrossRef]
- Humam, M.; Muñoz, O.; Christen, P.; Hostettmann, K. Tropane alkaloids of the aerial parts of Schizanthus tricolor. Nat. Prod. Commun. 2007, 2, 743–747. [Google Scholar]
- de Oliveira, S.L.; Tavares, J.F.; Castello Branco, M.V.S.; Lucena, H.F.S.; Barbosa-Filho, J.M.; Agra, M.d.F.; do Nascimento, S.C.; Aguiar, J.d.S.; da Silva, T.G.; de Simone, C.A.; et al. Tropane Alkaloids from Erythroxylum caatingae Plowman. Chem. Biodiv. 2011, 8, 155–165. [Google Scholar] [CrossRef]
- Galvez, E.; Martinez, M.; Trigo, G.G.; Florencio, F.; Vilches, J.; Garcia-Blanco, S.; Bellanato, J. Structural study of tropane-3-spiro-4'-imidazol-5'-one. J. Mol. Struct. 1981, 75, 241–254. [Google Scholar] [CrossRef]
- Bode, J.; Stam, C.H. The absolute configuration of the tropane alkaloid 6β,7β-epoxy-1αH,5αH-tropan-3α-y1(-)-2,3-dihydroxy-2-phenylpropionate from its n-butyl bromide. Acta Crystallogr., Sect. B: Struct. Crystallogr. Chryst. Chem. 1982, B38, 333–335. [Google Scholar] [CrossRef]
- Humam, M.; Christen, P.; Muñoz, O.; Hostettmann, K.; Jeannerat, D. Absolute configuration of tropane alkaloids bearing two α,β-unsaturated ester functions using electronic CD spectroscopy: application to (R,R)-trans-3-hydroxysenecioyloxy-6-senecioyloxytropane. Chirality 2008, 20, 20–25. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Muñoz, O.; Joseph-Nathan, P. Absolute configuration determination and conformational analysis of (-)-(3S,6S)-3alpha,6beta-diacetoxytropane using vibrational circular dichroism and DFT techniques. Chirality 2010, 22, 234–241. [Google Scholar]
- Bringmann, G.; Gunther, C.; Muhlbacher, J.; Lalith, M.D.; Gunathilake, P.; Wickramasinghe, A. Tropane alkaloids from Erythroxylum zeylanicum O.E. Schulz (Erythroxylaceae). Phytochemistry 2000, 53, 409–416. [Google Scholar] [CrossRef]
- Fodor, G.; Koczor, I.; Janzso, G. Stereochemistry of tropane alkaloids. XV. Partial synthesis of 6-hydroxyhyoscyamine. Arch.Pharm. (Weinheim, Ger.) 1962, 295, 91–95. [Google Scholar] [CrossRef]
- Fodor, G.; Soti, F. Correlation of valeroidine with (S)(-)-methoxysuccinic acid and of mono- and ditigloyltropane-3,6-diol with its (R)(+)antimer. Tetrahedron Lett. 1964, 5, 1917–1921. [Google Scholar] [CrossRef]
- Fodor, G.; Soti, F. The stereochemistry of the tropane alkaloids XVII. Correlation of valeroidine with (S)-(-)-methoxysuccinic acid and of mono- and ditigloyltropane-3,6-diol with its (R)-(+)-antimer. J. Chem. Soc. 1965, 6830–6833. [Google Scholar] [CrossRef]
- Fodor, G.; Vincze, I.W.; Toth, J. Stereochemistry of the tropane alkaloids. XIII. Absolute configuration and a simplified synthesis of valeroidine. J. Chem. Soc. 1961, 3219–3221. [Google Scholar]
- Glaser, R.; Peng, Q.J.; Perlin, A.S. Stereochemistry of the N-methyl group in salts of tropane alkaloids. J. Org. Chem. 1988, 53, 2172–2180. [Google Scholar] [CrossRef]
- Freedman, T.B.; Cao, X.; Dukor, R.K.; Nafie, L.A. Absolute configuration determination of chiral molecules in the solution state using vibrational circular dichroism. Chirality 2003, 15, 743–758. [Google Scholar] [CrossRef]
- Reina, M.; Burgueno-Tapia, E.; Bucio, M.A.; Joseph-Nathan, P. Absolute configuration of tropane alkaloids from Schizanthus species by vibrational circular dichroism. Phytochemistry 2010, 71, 810–815. [Google Scholar] [CrossRef]
- Dale, J.A.; Dull, D.L.; Mosher, H.S. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J. Org. Chem. 1969, 34, 2543–2549. [Google Scholar] [CrossRef]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Hoye, T.R.; Renner, M.K. Applications of MTPA (Mosher) Amides of Secondary Amines: Assignment of Absolute Configuration in Chiral Cyclic Amines. J. Org. Chem. 1996, 61, 8489–8495. [Google Scholar] [CrossRef]
- Butler, T.R. 10-Hydroxydarlingine, a New Tropane Alkaloid from the Australian Proteaceous Plant Triunia erythrocarpa. J. Nat. Prod. 2000, 65, 688–689. [Google Scholar] [CrossRef]
- Kusumi, T.; Fukushima, T.; Ohtani, I.; Kakisawa, H. Elucidation of the absolute configuration of amino acids and amines by the modified Mosher's method. Tetrahedron Lett. 1991, 32, 2939–2942. [Google Scholar]
- Latypov, S.K.; Seco, J.M.; Quinoa, E.; Riguera, R. MTPA vs. MPA in the Determination of the Absolute Configuration of Chiral Alcohols by 1H-NMR. J. Org. Chem. 1996, 61, 8569–8577. [Google Scholar] [CrossRef]
- Bieri, S.; Varesio, E.; Veuthey, J.-L.; Muñoz, O.; Tseng, L.-H.; Braumann, U.; Spraul, M.; Christen, P. Identification of isomeric tropane alkaloids from Schizanthus grahamii by HPLC-NMR with loop storage and HPLC-UV-MS/SPE-NMR using a cryogenic flow probe. Phytochem. Anal. 2006, 17, 78–86. [Google Scholar] [CrossRef]
- Humam, M.; Bieri, S.; Geiser, L.; Muñoz, O.; Veuthey, J.L.; Christen, P. Separation of four isomeric tropane alkaloids from Schizanthus grahamii by non-aqueous capillary electrophoresis. Phytochem. Anal. 2005, 16, 349–356. [Google Scholar] [CrossRef]
- Muñoz, O.; Piovano, M.; Garbarino, J.; Hellwing, V.; Breitmaier, E. Tropane alkaloids from Schizanthus litoralis. Phytochemistry 1996, 43, 709–713. [Google Scholar] [CrossRef]
- Muñoz, O.; Cortes, S. Tropane alkaloids from Schizanthus porrigens. Pharm. Biol. (London, U.K.) 1998, 36, 162–166. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Humam, M.; Shoul, T.; Jeannerat, D.; Muñoz, O.; Christen, P. Chirality and Numbering of Substituted Tropane Alkaloids. Molecules 2011, 16, 7199-7209. https://doi.org/10.3390/molecules16097199
Humam M, Shoul T, Jeannerat D, Muñoz O, Christen P. Chirality and Numbering of Substituted Tropane Alkaloids. Molecules. 2011; 16(9):7199-7209. https://doi.org/10.3390/molecules16097199
Chicago/Turabian StyleHumam, Munir, Tarik Shoul, Damien Jeannerat, Orlando Muñoz, and Philippe Christen. 2011. "Chirality and Numbering of Substituted Tropane Alkaloids" Molecules 16, no. 9: 7199-7209. https://doi.org/10.3390/molecules16097199