Antioxidant Activities of Melittis melissophyllum L. (Lamiaceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. In vitro experiments
| Extract | Et2O | CHCl3 | EtOAc | n-BuOH | H2O |
|---|---|---|---|---|---|
| Leaf | 0.79 | 1.14 | 1.31 | 0.41 | 1.98 |
| IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|
| Extract | Et2O | CHCl3 | EtOAc | n-BuOH | H2O | BHT |
| DPPH radical | 18.09 | 11.92 | 11.34 | 17.21 | 9.21 | 14.31 |
| O2°− radical | 8.11 | 7.91 | 14.42 | 13.26 | 6.38 | 10.46 |
| NO radical | 8.04 | 7.42 | 8.91 | 9.12 | 6.17 | 8.63 |

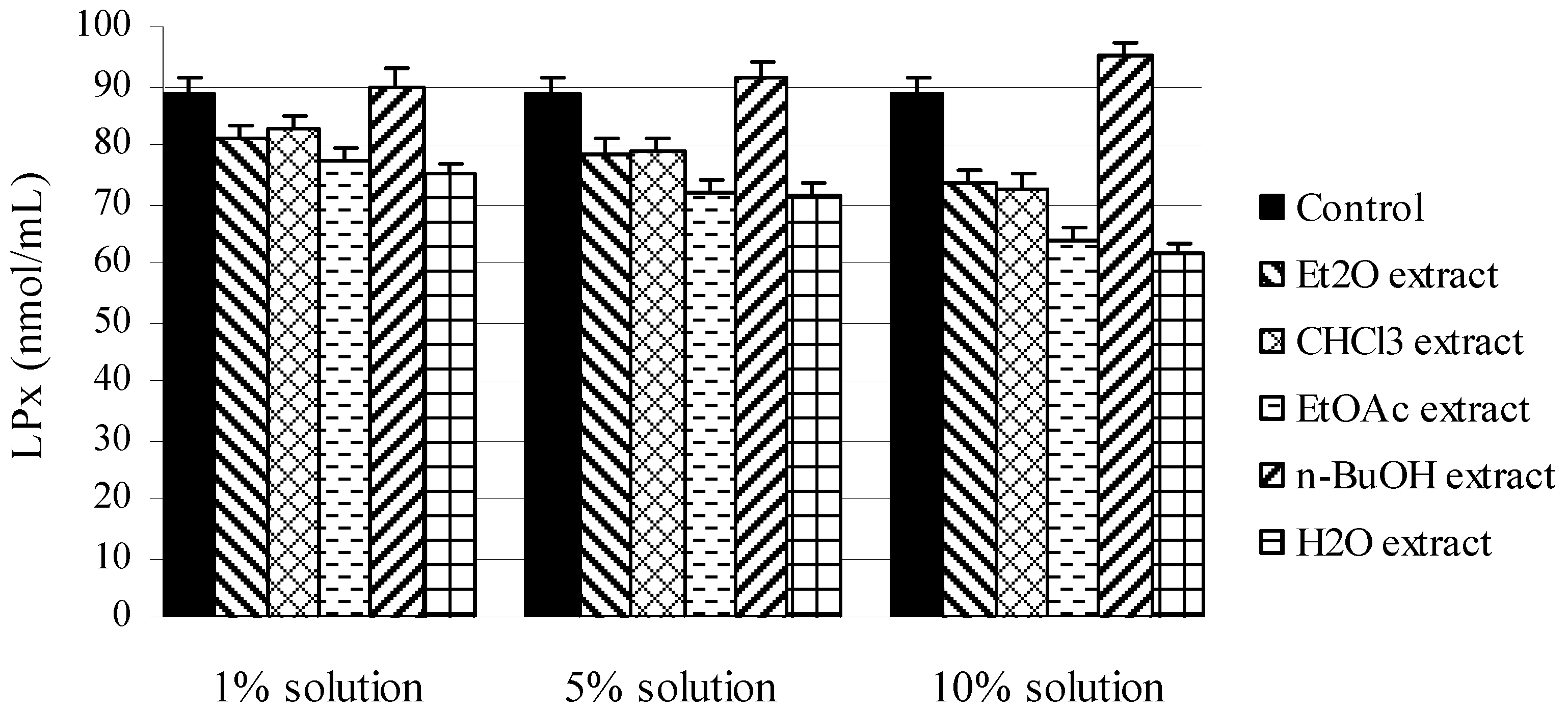
2.2. In vivo experiments
| Parameter | Control | Extract | ||||
|---|---|---|---|---|---|---|
| Et2O | CHCl3 | EtOAc | n-BuOH | H2O | ||
| GSHPx | 3.43 ± 0.17 | 3.96 ± 0.16a | 3.32 ± 0.18 | 2.84 ± 0.21a | 3.37 ± 0.15 | 3.46 ± 0.18 |
| GSH | 2.61 ± 0.13 | 2.76 ± 0.11 | 2.86 ± 0.14 | 2.12 ± 0.17a | 2.87 ± 0.22 | 3.49 ± 0.13a |
| GSHR | 3.96 ± 0.18 | 4.82 ± 0.21a | 5.36 ± 0.25a | 2.98 ± 0.14 a | 3.96 ± 0.19 | 5.52 ± 0.23a |
| Px | 4.38 ± 0.13 | 4.81 ± 0.17 a | 4.93 ± 0.21 a | 3.89 ± 0.13 a | 5.11 ± 0.25 a | 4.87 ± 0.15 a |
| LPx | 7.19 ± 0.23 | 7.36 ± 0.21 | 7.91 ± 0.19a | 6.71 ± 0.16 a | 7.12 ± 0.23 | 6.19 ± 0.27 a |
| CAT | 4.41 ± 0.16 | 3.83 ± 0.17 a | 5.03± 0.19a | 3.20 ± 0.15 a | 4.52 ± 0.11 | 5.49 ± 0.13a |
| XOD | 5.27 ± 0.17 | 6.17 ± 0.23a | 6.02 ± 0.16a | 4.23 ± 0.16 a | 5.11 ± 0.22 | 4.17 ± 0.19 a |
| Parameter | Control + CCl4 | Extract + CCl4 | ||||
|---|---|---|---|---|---|---|
| Et2O | CHCl3 | EtOAc | n-BuOH | H2O | ||
| GSHPx | 2.12 ± 0.17 | 2.31 ± 0.15 | 2.61 ± 0.18a | 1.62 ± 0.23a | 2.18 ± 0.24 | 2.58 ± 0.18a |
| GSH | 2.26 ± 0.17 | 2.18 ± 0.12 | 2.35 ± 0.19 | 1.86 ± 0.12 a | 1.92 ± 0.15 a | 2.49 ± 0.25 |
| GSHR | 2.53 ± 0.21 | 3.46 ± 0.28a | 4.25 ± 0.24a | 2.08 ± 0.15a | 2.26 ± 0.26 | 3.07 ± 0.26a |
| Px | 3.47 ± 0.18 | 3.05 ± 0.17a | 2.97 ± 0.25a | 2.84 ± 0.21a | 3.06 ± 0.23 | 3.01 ± 0.24a |
| LPx | 8.91 ± 0.29 | 7.12 ± 0.21a | 7.06 ± 0.24 a | 6.92 ± 0.17a | 6.98 ± 0.24 a | 6.81 ± 0.24a |
| CAT | 2.08 ± 0.17 | 2.17 ± 0.22 | 2.47 ± 0.25 | 1.02 ± 0.12a | 2.10 ± 0.14 | 1.44 ± 0.18a |
| XOD | 4.61 ± 0.25 | 3.69 ± 0.23a | 5.38 ± 0.21a | 3.02 ± 0.19a | 3.98 ± 0.17 a | 2.39 ± 0.14 a |
| Parameter | Control | Extract | ||||
|---|---|---|---|---|---|---|
| Et2O | CHCl3 | EtOAc | n-BuOH | H2O | ||
| GSHPx | 5.94 ± 0.22 | 4.81 ± 0.16a | 4.95 ± 0.19a | 2.77 ± 0.23a | 4.79 ± 0.16a | 3.89 ± 0.16a |
| GSH | 6.78 ± 0.12 | 6.22 ± 0.14a | 6.13 ± 0.17a | 5.56 ± 0.21a | 3.96 ± 0.21a | 4.37 ± 0.19 a |
| GSHR | 7.67 ± 0.24 | 6.17 ± 0.24a | 7.87 ± 0.27 | 5.56 ± 0.17a | 6.81 ± 0.19a | 7.72 ± 0.28 |
| Px | 3.72 ± 0.17 | 3.39 ± 0.21 | 3.69 ± 0.25 | 2.94 ± 0.19a | 3.46 ± 0.27 | 3.65 ± 0.18 |
| LPx | 4.81 ± 0.24 | 4.59 ± 0.28 | 3.78 ± 0.17a | 2.96 ± 0.13a | 4.74 ± 0.19 | 4.07 ± 0.24a |
| CAT | 4.28 ± 0.26 | 4.77 ± 0.18a | 3.75 ± 0.19a | 3.18 ± 0.16a | 4.11 ± 0.18 | 3.86 ± 0.23 |
| XOD | 4.76 ± 0.29 | 5.92 ± 0.31a | 5.44 ± 0.26a | 5.58 ± 0.19a | 5.41 ± 0.27a | 5.48 ± 0.24a |
| Parameter | Control + CCl4 | Extract + CCl4 | ||||
|---|---|---|---|---|---|---|
| Et2O | CHCl3 | EtOAc | n-BuOH | H2O | ||
| GSHPx | 6.08 ± 0.17 | 5.83 ± 0.21 | 5.77 ± 0.24 | 4.69 ± 0.21a | 4.94 ± 0.27a | 5.91 ± 0.33 |
| GSH | 5.21 ± 0.13 | 4.84 ± 0.19a | 3.97 ± 0.24a | 4.08 ± 0.17a | 3.03 ± 0.18a | 3.86 ± 0.24a |
| GSHR | 6.12 ± 0.29 | 5.76 ± 0.28 | 5.87 ± 0.22 | 4.12 ± 0.18a | 6.17 ± 0.29 | 6.58 ± 0.32 |
| Px | 4.08 ± 0.22 | 3.27 ± 0.16a | 4.86 ± 0.24a | 3.15 ± 0.23 a | 3.04 ± 0.18 a | 4.11 ± 0.29 |
| LPx | 5.11 ± 0.24 | 5.31 ± 0.17 | 4.92 ± 0.21 | 3.02 ± 0.24a | 5.17 ± 0.25 | 2.98 ± 0.12a |
| CAT | 3.74 ± 0.24 | 3.19 ± 0.23a | 3.24 ± 0.19a | 3.07 ± 0.25a | 2.98 ± 0.18a | 3.13 ± 0.28a |
| XOD | 6.19 ± 0.31 | 6.08 ± 0.26 | 4.96 ± 0.24a | 5.11 ± 0.17a | 6.97 ± 0.25a | 5.23 ± 0.21a |
3. Experimental
3.1. General
3.2. In vitro experiments
3.3. In vivo antioxidant activity
3.4. Chemicals
3.5. Statistical analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Halliwell, B. Antioxidants and Human Disease: A general Introduction. Nutr. Rev. 1997, 55, 44–52. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D. In vitro antioxidant studies of Annoasquamosa Linn. Leaves. Leaves. Ind. J. Exp. Biol. 2004, 42, 803–807. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1986; pp. 183–189. [Google Scholar]
- Blumenthal, M. The Complete German Commission E Monographs; American Botanical Council: Austin, TX, USA, 1999. [Google Scholar]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed; Intercept Ltd.: London, UK, 1999. [Google Scholar]
- Velasco-Negueruela, A.; Sanz, J.; Perez-Alonso, M.J.; Pala-Paul, J. The volatile components of the aerial parts of Melittis melissophyllum L. Bot. Complutensis 2004, 28, 133–136. [Google Scholar]
- Skrzypczak-Pietraszek, E.; Hensel, A. Polysaccharides from Melittis melissophyllum L. herb and callus. Pharmazie 2000, 55, 768–771. [Google Scholar]
- Skrzypczak, E.; Skrzypczak, L. The tissue culture and chemical analysis of Melittis melissophyllum L. Acta Hort. 1993, 330, 263–267. [Google Scholar]
- Nuutila, A.M.; Puupponen-Pimia, R.; Aarni, M.; Oksman-Caldentey, K.M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Fejes, S.; Blazovics, A.; Lemberkovics, E.; Petri, G.; Szoke, E.; Kery, A. Free radical scavenging and membrane protective effects of methanol extracts from Anthriscus cerefolium L. (Hoffm.) and Petroselinum crispum (Mill.) Nym. ex A. W. Hill. Phytother. Res. 2000, 14, 362–365. [Google Scholar] [CrossRef]
- Fraga, B. Natural sesquiterpenoids. Nat. Prod. Rep. 2003, 20, 392–413. [Google Scholar] [CrossRef]
- Fischer, H.; Lu, T.; Cantrell, L.; Castaneda-Acosta, J.; Quijano, L.; Franzblau, G. Antimycobacterial evaluation of germacranolides. Phytochemistry 1988, 49, 559–564. [Google Scholar]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subject. Br. J. Nutr. 1999, 8, 447–455. [Google Scholar]
- Mann, J. Secondary Metabolism, 2nd ed; Oxford University Press: Oxford, UK, 1992; pp. 279–280. [Google Scholar]
- Balanehru, S.; Nagarajan, B. Protective effect of oleanolic acid and ursolic acid against lipid peroxidation. Biochem. Int. 1991, 24, 981–990. [Google Scholar]
- Kitani, K.; Kanai, S.; Ivy, G.O.; Carillo, M.C. Pharmacological modifications of endogenous antioxidant enzymes with special reference to the effects of deprenyl: A possible antioxidant strategy. Mech. Age. Dev. 1999, 111, 211–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals; Scientific Publisher: Stuttgart, Germany, 1994; p. 446. [Google Scholar]
- Williams, C.A.; Goldstone, A.; Greenham, J. Flavonoids, cinnamic-acid, and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef]
- Cock, C.; Samman, S. Flavonoids chemistry metabolism, cardioprotectioeffects and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Shetty, K. Biotechnology to harness the benefits of dietary phenolics; focus on Lamiaceae. Asia Pacific J. Clin. Nutr. 1997, 6, 162–171. [Google Scholar]
- Doba, T.; Burton, G.W.; Ingold, K.U. Antioxidant and co-oxidant activity of vitamin C. The effects of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multimalleral phospholipid liposomes. Biochim. Biophys. Acta 1985, 835, 298–303. [Google Scholar] [CrossRef]
- Decker, E.A. Phenolics: prooxidants or antioxidants? Nutr. Rev. 1997, 55, 396–407. [Google Scholar] [CrossRef]
- Biaglow, J.E.; Varnes, M.E.; Epp, E.R.; Clark, E.P.; Tuttle, S.; Held, K.D. Cellular protection against damage by hydroperoxides: Role of glutatione. In Oxygen Radicals in Biology and Medicine; Simic, M.G., Taylor, K.A., Ward, J.F., von Sonntag, C., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 567–573. [Google Scholar]
- Yu, J.; Taylor, K.E.; Zou, H.; Biswas, N.; Bewtra, J.K. Phenol conversion and dimeric intermediates in horseradish peroxidase-catalysed phenol removal from water. Environ. Sci. Technol. 1994, 28, 2154–2160. [Google Scholar] [CrossRef]
- Handa, S.S.; Sharma, A.; Chakraborti, K.K. Natural products in plants as liver protecting drugs. Fitoterapia 1986, 57, 307–310. [Google Scholar]
- Cholbi, M.R.; Paya, M.; Alcaraz, M.J. Inhibitory effect of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experimentia 1991, 47, 195–198. [Google Scholar] [CrossRef]
- Sousa, R.L.; Marletta, M.A. Inhibition of cytochrome P-450 activity in rat liver microsomes by the natural occuring flavonoid, quercetin. Arch. Biochem. Biophys. 1985, 240, 345–348. [Google Scholar] [CrossRef]
- Rekka, E.; Kourounakis, P.N. Effect of hydroxyethyl rutoside and related compounds on lipid peroxidation and free radical scavening activity. Some structural aspects. J. Pharmac. Pharmacol. 1991, 43, 486–490. [Google Scholar] [CrossRef]
- Swei, A.; Suzuki, H.; Parks, D.A.; Delano, F.A.; Schmid-Schonbein, G.W. Mechanism of oxygen free radical formation in experimental forms of hypertension. INABIS 1998, 1, 837. [Google Scholar]
- Desco, M.; Asensi, M.; Marquez, R.; Martinez-Valls, J.; Vento, M.; Pallardo, F.V.; Sastre, J.; Viña, J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 2002, 51, 1118–1124. [Google Scholar] [CrossRef]
- Giler, S.; Sperling, O.; Brosh, S.; Urca, I.; de Vries, A. Serum xanthine oxidase in jaundice. Clin. Chim. Acta 1975, 63, 37–40. [Google Scholar] [CrossRef]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef]
- Kisker, C.; Schindelin, H.; Rees, D.C. Molybdenum-cofactor-containig enzymes: structure and mechanism. Annu. Rev. Biochem. 1997, 66, 233–267. [Google Scholar] [CrossRef]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDRc for Herbal Medicines; Jaenicke, C., Ed.; Medical Economics Co: Montvale, NJ, USA, 2000; pp. 271-275, 719-725. [Google Scholar]
- Sanchez-Moreno, C.; Larrauri, A.; Saura-Calixto, F. A procedure to measure their anti-radical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–2768. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Callome, M.; Hu, P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinick, J.; Van den Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of Xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Green, C.; Wagner, A.; Glogowski, J.; Skipper, I.; Wishnok, S.; Tannenbaum, R. Analysis of nitrat, nitrit and [15N]nitrit in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar]
- Babu, H.; Shylesh, S.; Padikkala, J. Antioxidant and hepatoprotective effect of Acanthu silicifolius. Fitoterapia 2001, 72, 272–277. [Google Scholar] [CrossRef]
- Cheesman, H.; Beavis, A.; Eserbauer, H. Hydroxyl-radical-induced-iron-catalysed degration of 2-deoxyribose. Biochem. J. 1988, 252, 649–653. [Google Scholar]
- Fukuzawa, K.; Seko, T.; Minami, K.; Terao, J. Dynamics of iron-ascorbate-induced lipid peroxidation in charged and uncharged phospholipid vesicles. Lipids 1993, 28, 497–503. [Google Scholar] [CrossRef]
- Buege, A.J.; Aust, D.S. Methods in Enzymology; Fleischer, S., Parker, L., Eds.; Academic Press: New York, NY, USA, 1988; pp. 302–310. [Google Scholar]
- Simon, L.M.; Fatrai, Z.; Jonas, D.E.; Matkovics, B. Study of metabolism enzymes during the development of Phaseolus vulgaris. Biochem. Physiol. Plant 1974, 166, 389–393. [Google Scholar]
- Beers, R.F.J.; Sizer, J.W. Spectrophotometric method for measuring of breakdown of hydrogen peroxide by Catalase. J. Biol. Chem. 1950, 195, 133–140. [Google Scholar]
- Chin, P.T.Y.; Stults, F.H.; Tappel, A.L. Purification of rat lung soluble Glutathione Peroxidase. Biochem. Biophys. Acta 1976, 445, 558–660. [Google Scholar] [CrossRef]
- Glatzle, D.; Vuillenmir, K.; Weber, F.; Decker, K. Glutathione reductase test with whole blood a convenient procedure for the assessment of the riboflavine status in human. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef]
- Bergmayer, U.H. Methoden Der Enzymatischen Analyse; Verlag Chemie: Weinheim, Germany, 1970. [Google Scholar]
- Kapetanović, I.M.; Mieyal, I.I. Inhibition of acetaminophen induced hepatotoxicity by phenacetin and its alkoxy analogs. J. Pharmacol. Exp. Ther. 1979, 209, 25–30. [Google Scholar]
- Gornall, H.G.; Nardwall, C.L. Estimation of total protein in tissue homogenate. J. Biol. Chem. 1949, 177, 751–756. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Raseta, M. Antioxidant Activities of Melittis melissophyllum L. (Lamiaceae). Molecules 2011, 16, 3152-3167. https://doi.org/10.3390/molecules16043152
Kaurinovic B, Popovic M, Vlaisavljevic S, Raseta M. Antioxidant Activities of Melittis melissophyllum L. (Lamiaceae). Molecules. 2011; 16(4):3152-3167. https://doi.org/10.3390/molecules16043152
Chicago/Turabian StyleKaurinovic, Biljana, Mira Popovic, Sanja Vlaisavljevic, and Milena Raseta. 2011. "Antioxidant Activities of Melittis melissophyllum L. (Lamiaceae)" Molecules 16, no. 4: 3152-3167. https://doi.org/10.3390/molecules16043152
APA StyleKaurinovic, B., Popovic, M., Vlaisavljevic, S., & Raseta, M. (2011). Antioxidant Activities of Melittis melissophyllum L. (Lamiaceae). Molecules, 16(4), 3152-3167. https://doi.org/10.3390/molecules16043152







