Abstract
Synthesis of two ent-kaurane diterpene glycosides, steviol 19-O-β-D-glucopyranosiduronic acid (steviol glucuronide, 5 ), and 13-hydroxy ent-kaur-16-en-19-oic acid-β-D-glucopyranosyl ester (7) has been achieved from a common starting material, steviol, using phase transfer catalyst. Also, synthesis of an additional 17-nor-ent-kaurane glycoside, namely 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-D-glucopyranosyl ester (10) was performed using the starting material isosteviol and similar synthetic methodology. Synthesis of all three steviol glycosides was performed using straightforward chemistry and their structures were characterized on the basis of 1D and 2D NMR as well as mass spectral (MS) data.
1. Introduction
The major constituents isolated from the leaves of Stevia rebaudiana Bertoni (family: Asteraceae) are the potently sweet diterpenoid glycosides stevioside, and rebaudioside A. These compounds which are known as Stevia sweeteners are glycosides of the diterpene steviol, ent-13-hydroxykaur-16-en-19-oic acid [1]. Stevioside tastes about 150-250 times sweeter than sucrose, whereas rebaudioside A tastes about 200-300 times sweeter than sucrose; both are non-caloric. In some parts of the world, including Japan, South Korea, Israel, Mexico, Paraguay, Brazil, Argentina, and Switzerland, these steviol glycosides are used to sweeten food products and beverages. As a part of our continuing research to discover natural sweeteners, we have reported the isolation of several glycosides from the commercial extract of S. rebaudiana [2,3,4,5,6,7,8]. Apart from isolating novel compounds from S. rebaudiana and utilizing them as possible natural sweeteners or sweetness enhancers, we are also engaged in understanding the physicochemical profiles of steviol glycosides in various systems of interest and their metabolites, as well as their characterization [9]. Though many steviol glycosides have been reported in the literature, focused synthetic studies were not carried out. In this article, we present the synthesis of three ent-kaurane diterpene glycosides possessing steviol and isosteviol skeletons, and the characterization of their structures based on extensive NMR and mass spectroscopic data.
2. Results and Discussion
2.1. Chemistry
The two compounds 5 and 7 were synthesized via the common intermediate 13-acetyloxy-ent-kaur-16-en-19-oic acid (steviol acetate, 2), prepared by acetylation of steviol (1) with Ac2O and pyridine (Scheme 1).
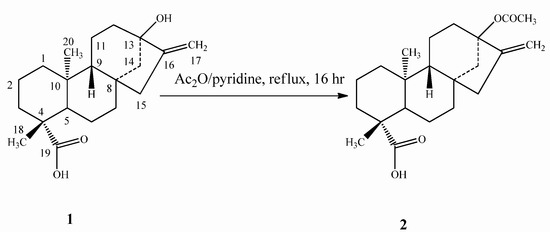
Scheme 1.
Synthesis of steviol acetate (2).
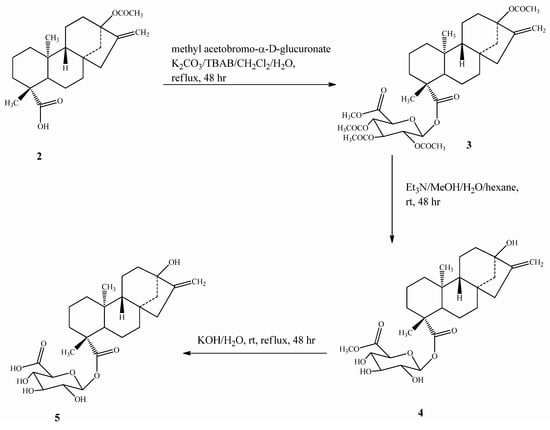
Scheme 2.
Synthesis of steviol glucuronide (5).
Compound 5 was prepared by esterification of 2 with bromo-2,3,4-tri-O-acetyl-α-D-glucopyran-uronic acid methyl ester (methyl acetobromo-α-D-glucuronate) in the presence of the phase transfer catalyst tetrabutylammonium bromide (TBAB) to yield 3, which upon deacetylation using Et3N, followed by alkaline hydrolysis, furnished the final steviol glucuronide (5) (Scheme 2). Compound 7 was synthesized from 2 using the same esterification procedure as mentioned for 5 with 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide(acetobromo-α-D-glucose) to furnish intermediate 6, which on deacetylation using Et3N afforded the final compound 7 (Scheme 3).
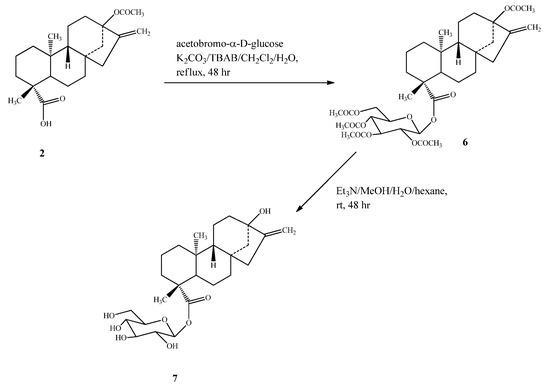
Scheme 3.
Synthesis of 13-hydroxy ent-kaur-16-en-19-oic acid-β-D-glucopyranosyl ester (7).
Compound 10 was prepared by esterification of isosteviol (8) with acetobromo-α-D-glucose in the presence of phase transfer catalyst as described for 7 , producing intermediate 9, which on deacetylation with Et3N as reported above yielded compound 10 (Scheme 4).
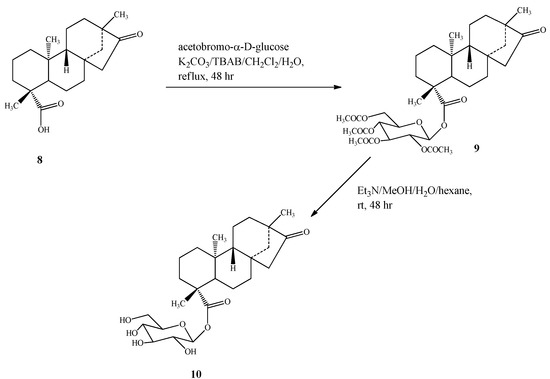
Scheme 4.
Synthesis of 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-D-gluco-pyranosyl ester (10).
2.2. Spectroscopy
The structural characterization of 5 , 7 and 10 was performed on the basis of one dimensional (1H, 13C), two-dimensional (1H-1H COSY, 1H-13C HMQC, 1H-13C HMBC) NMR and mass spectral data, as well as in comparison with literature values [3,10]. The attachment of sugars at the C-19 position of the steviol skeleton in 5 and 7 , and the isosteviol skeleton in 10 was characterized by the key HMBC correlation of the anomeric protons of the respective sugar moieties with the C-19 carbonyl group. The 1H- and 13C-NMR values for all the protons and carbons were assigned on the basis of COSY, HMQC and HMBC correlations and are given in Table 1 and Table 2. The anomeric protons in all three glycosides 5, 7 and 10 were observed as doublets at δ 5.44 (d, 7.8 Hz), 5.41 (d, 8.2 Hz) and 5.38 (d, 8.2 Hz) respectively, suggesting their β-configuration similar to that of the steviol glycosides isolated from S. rebaudiana [2,3,4,5,6,7,8]. This suggested that even though esterification was performed using their corresponding α-derivatives; the configuration of the final products 5 , 7 , and 10 was observed to correspond to their β-derivatives; an identical phenomenon was reported earlier for glycosylation of triterpene acids under phase transfer catalytic conditions [11].

Table 1.
1H-NMR chemical shift values for compounds 5 , 7 and 10 * recorded in CD3OD a.
| Position | 5 | 7 | 10 |
|---|---|---|---|
| 1 | 0.86 (m, 1H), 1.86 (m, 1H) | 0.86 (m, 1H), 1.88 (m, 1H) | 0.96 (m, 1H), 1.68 (m, 1H) |
| 2 | 1.39 (m, 1H), 1.90 (m, 1H) | 1.43 (m, 1H),1.93 (m, 1H) | 1.36 (m, 1H), 1.92 (m, 1H) |
| 3 | 1.02 (m, 1H), 2.26 (d, 11.9, 1H) | 1.06 (m, 1H), 2.19 (d, 12.4, 1H) | 1.05 (m, 1H), 2.16 (d, 13.2, 1H) |
| 5 | 1.08 (m, 1H) | 1.12 (m, 1H) | 1.20 (m, 1H) |
| 6 | 1.82 (m, 1H), 1.93 (m, 1H) | 1.85 (m, 1H), 1.96 (m, 1H) | 1.86 (m, 2H) |
| 7 | 1.45 (m, 1H), 1.56 (m, 1H) | 1.44 (m, 1H), 1.54 (m, 1H) | 1.47 (m, 1H), 1.66 (m, 1H) |
| 9 | 0.88 (m, 1H) | 0.96 (m, 1H) | 1.24 (m, 1H) |
| 11 | 1.60 (m, 1H), 1.79 (m, 1H) | 1.61 (m, 1H), 1.77 (m, 1H) | 1.25 (m, 1H), 1.70 (m, 1H) |
| 12 | 1.53 (m, 1H), 1.87 (m, 1H) | 1.46 (m, 1H), 1.74 (m, 1H) | 1.42 (m, 1H), 1.56 (m, 1H) |
| 14 | 1.58 (m, 1H), 2.23 (d, 12.1, 1H) | 1.28 (m, 1H), 2.11 (m, 1H) | 1.44 (m, 1H), 1.58 (m, 1H) |
| 15 | 2.02 (m, 1H), 2.16 (d, J = 17.4, 1H) | 2.06 (m, 1H), 2.17 (m, 1H) | 1.80 (m, 1H), 2.65 (dd, 3.1, 17.2, 1H) |
| 17 | 4.59 (s, 1H), 4.78 (br s, 1H) | 4.77 (br s, 1H), 4.93 (s, 1H) | |
| 18 | 1.23 (s, 3H) | 1.20 (s, 3H) | 1.24 (s, 3H) |
| 20 | 0.97 (s, 3H) | 0.98 (s, 3H) | 0.93 (s, 3H) |
| 1′ | 5.44 (d, 7.8, 1H) | 5.41 (d, J = 8.2 Hz, 1H) | 5.38 (d, 8.2, 1H) |
| 2′ | 3.60 (dd, 8.2, 9.1, 1H) | 3.35 (dd, 7.1, 7.6, 1H) | 3.32 (dd, 7.2, 7.8, 1H) |
| 3′ | 3.42 (dd, 8.1, 8.9, 1H) | 3.44 (dd, 8.3, 9.1, 1H) | 3.43 (dd, 8.1, 9.1, 1H) |
| 4′ | 3.49 (dd, 8.1, 9.2, 1H) | 3.34 (dd, 8.2, 9.4, 1H) | 3.34 (dd, 8.1, 9.1, 1H) |
| 5′ | 3.72 (d, 8.2, 1H) | 3.39 (ddd, 8.1, 2.1, 7.4, 1H) | 3.37 (ddd, 8.2, 1.9, 7.2, 1H) |
| 6′ | 3.66 (dd, 2.1, 12.1, 1H), 3.81 (dd, 4.2, 12.1, 1H) | 3.68 (dd, 1.9, 12.1, 1H), 3.82 (dd, 3.9, 12.1, 1H) |
a assignments made on the basis of COSY, HMQC and HMBC correlations; b Chemical shift values are in δ (ppm); c Coupling constants are in Hz; * δH 0.80 (s, 3H): CH3-13 for compound 10 .

Table 2.
13C-NMR chemical shift values for 5 , 7 and 10 * recorded in CD3OD a.
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 42.0 | 41.5 | 39.5 |
| 2 | 20.3 | 20.2 | 19.1 |
| 3 | 39.2 | 38.6 | 38.0 |
| 4 | 45.2 | 45.1 | 43.9 |
| 5 | 58.7 | 58.5 | 57.6 |
| 6 | 23.1 | 22.8 | 20.7 |
| 7 | 42.8 | 42.4 | 41.4 |
| 8 | 42.9 | 43.0 | 48.4 |
| 9 | 55.5 | 55.0 | 53.9 |
| 10 | 40.9 | 40.5 | 37.6 |
| 11 | 21.5 | 21.3 | 21.1 |
| 12 | 40.7 | 40.4 | 38.6 |
| 13 | 81.0 | 88.5 | 39.1 |
| 14 | 47.4 | 47.2 | 54.6 |
| 15 | 48.8 | 48.6 | 48.3 |
| 16 | 157.2 | 157.0 | 223.8 |
| 17 | 103.5 | 103.4 | |
| 18 | 29.1 | 29.1 | 27.6 |
| 19 | 178.2 | 178.0 | 176.8 |
| 20 | 16.5 | 16.3 | 19.1 |
| 1′ | 95.6 | 95.6 | 95.5 |
| 2′ | 78.7 | 74.0 | 73.8 |
| 3′ | 74.0 | 77.7 | 77.3 |
| 4′ | 73.6 | 71.0 | 70.8 |
| 5′ | 77.7 | 77.8 | 77.6 |
| 6′ | 177.5 | 61.2 | 61.1 |
a assignments made on the basis of HMQC and HMBC correlations; b Chemical shift values are in δ (ppm); * δC 12.8: CH3-13 for compound 10 .
3. Experimental
3.1. General
Melting points were measured using a SRS Optimelt MPA 100 instrument and are uncorrected. Optical rotations were recorded using a Rudolph Autopol V at 25 °C and IR spectral data was acquired using a Perkin Elmer 400 Fourier Transform Infrared (FT-IR) spectrometer equipped with a universal attenuated total reflectance (UATR) polarization accessory. NMR spectra were acquired on Bruker Avance DRX 500 MHz using standard pulse sequences. Chemical shifts are given in δ (ppm), and coupling constants are reported in Hz. HRMS and MS/MS data were generated with a Waters Premier Quadrupole Time-of-Flight (Q-TOF) mass spectrometer equipped with an electrospray ionization source operated in the positive-ion mode and ThermoFisher Discovery OrbiTrap in the positive mode of electrospray. Samples were diluted with water: acetonitrile (1:1) containing 0.1% formic acid and introduced via infusion using the onboard syringe pump. HPLC was performed on an Agilent 1100 system using a Phenomenex Prodigy ODS (3) column (250 × 21.2 mm, 5 μm) or a Beckman Gold system with a Zorbax amino (150 × 4.6 mm, 5 μm) column.
3.2. Isolation
Purification of compound 5 was carried out using the Beckman HPLC system using an isocratic solvent method; UV Detection: 210 nm; Mobile Phase A: 75% CH3CN/25% H2O/0.05% AcOH; Mobile Phase B: 100% CH3CN/0.05% AcOH; Flow Rate: 1.0 mL/min; Gradient ratio: Mobile Phase A/B (60:40), by collecting the peak eluting at tR 1.33 min. Compound 7 was purified by using the Agilent HPLC 1100 system. UV Detection: 210 nm; Mobile Phase A: H2O (0.02% AcOH, 0.08% NH4OAc); Mobile Phase B: CH3CN; using gradient [25% B for 8.5 min, 25 to 29% B over 1.5 min, 29 to 30% B over 5.5 min, 30 to 34% B over 4.0 min, 34% B for 6 min, 34 to 52% B over 2.0 min, 52% B for 3.0 min, 52 to 70% B over 1.0 min, 70% B for 5.5 min] at 5 mL/min flow rate. The peak eluting at tR 28.3 min corresponds to 7. Compound 10 was also purified using the Agilent HPLC 1100 system. UV Detection: 226 nm; Mobile Phase A: H2O (0.1% TFA); Mobile Phase B: CH3CN; gradient increased from 95:5 (A:B) to 0:100 (A:B) over 30 min; Flow Rate: 5.0 mL/min. The peak eluting at tR 18.2 min furnished 10.
Steviol acetate (2): Steviol acetate (2) was prepared from steviol (1) as reported in the literature and characterized based on the 1H-NMR and mass spectral data and comparison with the spectral data reported in the literature [12]. 1H-NMR (500 MHz, CDCl3): δ 4.92 (s, 1H, 17-H), 4.86 (s, 1H, 17-H), 2.03 (s, 3H, OCOCH3); HRMS (M+Na)+m/z 383.2198 (calcd. for C22H32O4Na: 383.2208).
Steviol glucuronide (5): Compound 2 (0.633 g, 1.76 mmol) was dissolved in CH2Cl2 (25 mL) and distilled water (3 mL). TBAB (10 mg), K2CO3 (0.62 g, 4.50 mmol), and methyl acetobromo-α-D-glucuronate (0.80 g, 2.02 mmol) were added at room temperature. The reaction mixture was refluxed for 48 h, cooled to room temperature and the layers were separated. The aqueous layer was extracted with additional amounts of CH2Cl2 (2 × 20 mL) and the combined organic layer was washed with water (2 × 30 mL) and brine (40 mL). Concentration of the CH2Cl2 layer under vacuum furnished a residue (1.05 g), which showed a peak at m/z 677 in its mass spectrum corresponding to the (M+H)+ ion of 3. Compound 3 (1.00 g, 14.7 mmol) was dissolved in 10% solution of Et3N in MeOH-H2O-hexane (10:2:1, 23 mL) and stirred at room temperature for 48 h. The MeOH and hexane were evaporated under vacuum and the resulting product (0.73 g) was identified as 4 on the basis of its mass spectrum, which showed a peak at m/z 509 corresponding to its (M+H)+ ion. Compound 4 (0.70 g) was dissolved in H2O (20 mL) and KOH (0.16 g, 0.28 mmol) was added. The mixture was stirred at room temperature for 48 h and the mixture was subjected to evaporation under reduced pressure. Purification of the residue obtained after evaporation was performed using HPLC furnished 420 mg of 5. White powder, mp 198.7 °C, [α]D25 −70.6 (c 1.0, MeOH); IR νmax: 3303, 2937, 1723, 1599, 1053, 970 cm−1; 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M+NH4)+m/z 512.2852 (calcd. for C26H42O9N: 512.2860); (M+Na)+m/z 517.2405 (calcd. for C26H38O9Na: 517.2414).
13-Hydroxy-ent-kaur-16-en-19-oic acid-β- D-glucopyranosyl ester (7): To a solution of 2 (2.00 g, 5.56 mmol) in CH2Cl2 (35 mL) and distilled water (4 mL); TBAB (30 mg), K2CO3 (1.94 g, 14.06 mmol), and acetobromo-α-D-glucose (2.88 g, 7 mmol) were added at room temperature. The reaction mixture was refluxed for 48 h, cooled to room temperature and the layers were separated. The aqueous layer was extracted with additional CH2Cl2 (2 × 50 mL) and the combined organic layer was washed with water (2 × 60 mL) and brine (100 mL). Concentration of the CH2Cl2 layer under vacuum furnished a residue (2.23 g) which was identified as 6 on the basis of its EIMS spectrum which showed a peak at m/z 691 corresponding to its (M+H)+ ion. Compound 6 (2.00 g, 2.90 mmol) was dissolved in 10% solution of Et3N in MeOH-H2O-hexane (10:2:1, 45 mL) and stirred at room temperature for 48 h. The residue resulting after evaporation of the MeOH and hexane was purified by HPLC to furnish 850 mg of 7. White powder, [α]D25 −62.4 (c 1.0, EtOH); IR νmax: 3350, 2929, 1725, 1033, 890 cm−1; 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M+Na)+m/z 503.2608 (calcd. for C26H40O8Na: 503.2621).
13-Methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β- D-glucopyranosyl ester (10): To a solution of 8 (0.70 g, 2.20 mmol) in CH2Cl2 (15 mL) and distilled water (4 mL) was added TBAB (15 mg), K2CO3 (1.00 g, 7.25 mmol), and acetobromo-α-D-glucose (1.25 g, 3.04 mmol). The reaction mixture was refluxed for 48 h, cooled to room temperature and worked-up as described above to furnish a residue (1.20 g) which showed a peak at m/z 649 in its EIMS spectrum corresponding to the (M+H)+ ion of 9. Compound 9 (1.00 g, 1.54 mmol) was deacetylated as described above and upon usual work-up afforded a residue which was purified by HPLC to yield 500 mg of 10. White powder, mp 172.5 °C, [α]D25 −55.8 (c 1.0, EtOH); IR νmax: 3352, 2928, 1726, 1034, 891 cm−1; 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M+Na)+m/z 503.2611 (calcd. for C26H40O8Na: 503.2621).
4. Conclusions
In conclusion, three ent-kaurane diterpene glycosides 5, 7 and 10 were synthesized from the natural products steviol and isosteviol through simplified procedures. To the best of our knowledge, this is the first report of the synthesis of C-19 glycosidic linkages on the steviol and istosteviol skeletons. The structures of all the synthesized compounds were characterized on the basis of NMR (1D and 2D) and mass spectral data, as well as in comparison with the data reported in the literature.
Acknowledgements
We wish to thank AMRI, Bothell Research Center, Bothell, WA for obtaining selected NMR spectral data and Chris Mubarak, Analytical Sciences Department, The Coca-Cola Company, Atlanta, GA for providing IR spectral data.
- Samples Availability: Samples of the three synthesized steviol glycosides 5 , 7 , and 10 are available from the authors.
References
- Brandle, J.E.; Starrratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. A new diterpenoid glycoside from Stevia rebaudiana. Molecules 2011, 16, 2937–2943. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Structures of the novel diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 1057–1060. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Additional minor diterpene glycosides from Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 1059–1062. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpene glycosides from the leaves of Stevia rebaudiana. Phytochemistry Lett. 2011, 4, 209–212. [Google Scholar]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Structures of the novel α-glucosyl linked diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 2034–2038. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Stability study of steviol glycosides in mock beverages using fluorescent light exposure under ICH guidelines. Int. J. Pharm. Pharm. Sci. 2011, 3, 316–323. [Google Scholar]
- Geuns, J.M.C.; Buyse, J.; Vankeirsblick, A.; Temme, E.H.M.; Compernolle, F.; Toppet, S. Identification of steviol glucuronide in human urine. J. Agric. Food Chem. 2008, 56, 8507–8513. [Google Scholar] [CrossRef]
- Bliard, C.; Massiot, G.; Nazabadioko, S. Glycosylation of acids under phase transfer conditions; Partial synthesis of saponins. Tetrahedron Lett. 1994, 35, 6107–6108. [Google Scholar] [CrossRef]
- Gianfagna, T.; Zeevaart, J.A.D.; Lusk, W.J. Synthesis of [2H] gibberellins from steviol using the fungus Gibberella fujikuroi. Phytochemistry 1983, 22, 427–430. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).