Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006
Abstract
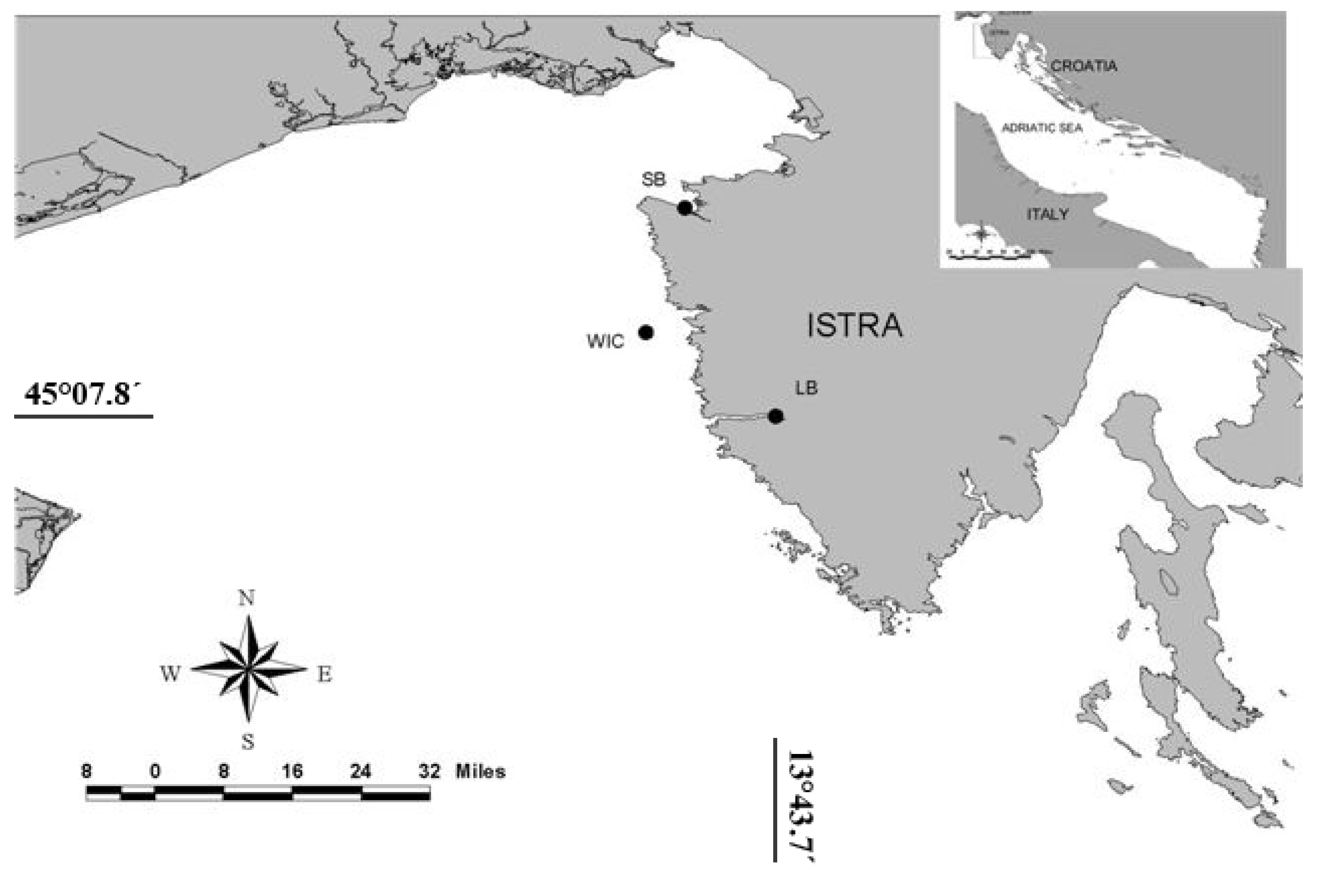
:1. Introduction
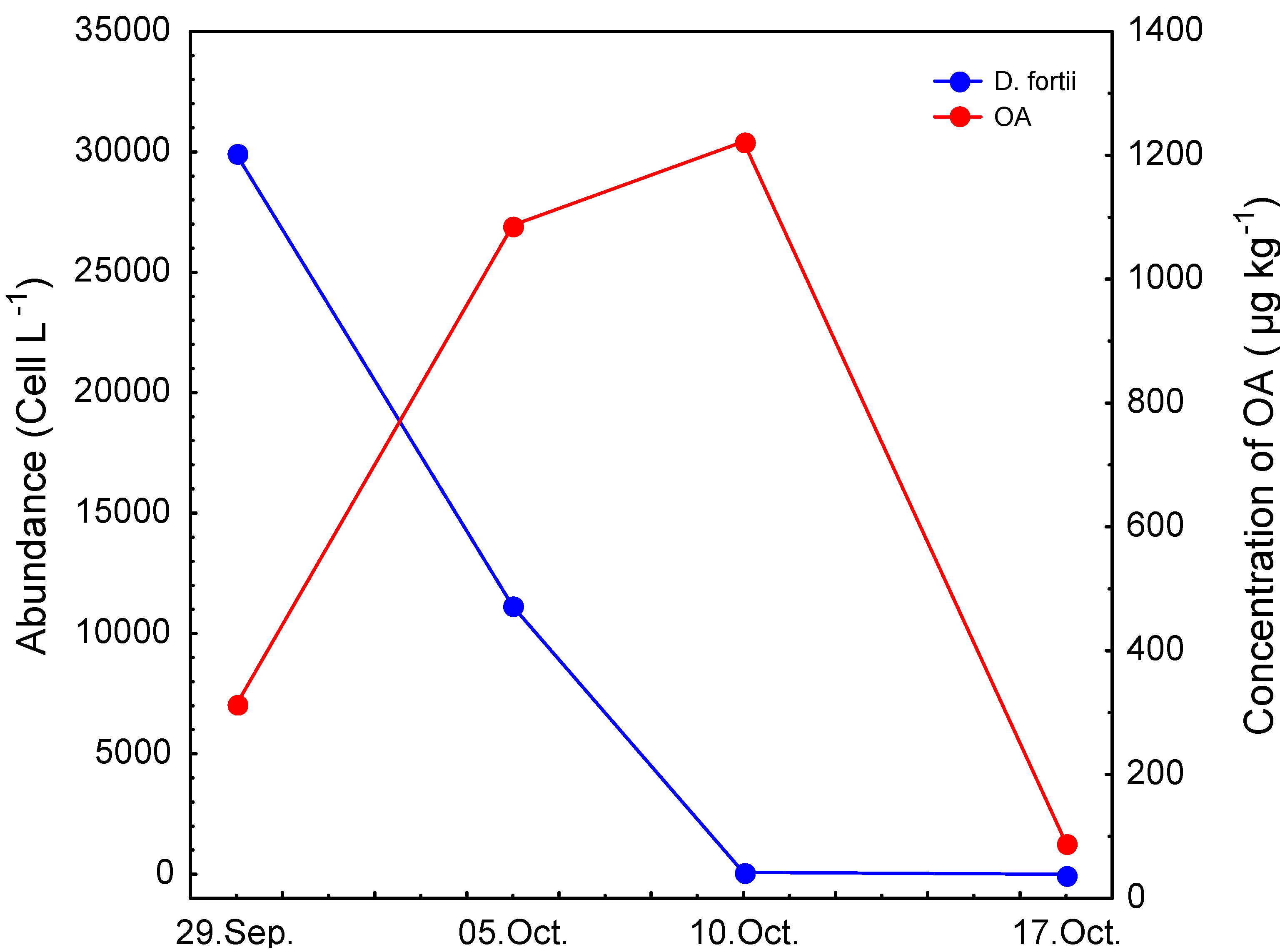
2. Results and Discussion
| Station | Date | Mortality | Survival times (h) |
|---|---|---|---|
| SB | 09 October | 3/3 | 3.00, 3.00, 4.17 |
| SB | 16 October | 3/3 | 4.00, 4.00, 5.00 |
| SB | 23 October | 2/3 | 3.00, 3.00 |
| WIC | 23 August | 3/3 | 0.02, 0.04, 0.15 |
| LB | 29 September | 3/3 | 3.00, 3.25, 3.33 |
| LB | 05 October | 3/3 | 1.17, 1.17, 1.25 |
| LB | 10 October | 3/3 | 2.00, 2.00, 3.00 |
| LB | 17 October | 2/3 | 4.00, 4.17 |
| Station | Date | Free OA (µg/kg) | OA esters (µg/kg) | µg total OA eq./kg | OA esters (%) | homoYTX(mg/kg) | PTX-2-SA (μg/kg) |
|---|---|---|---|---|---|---|---|
| SB | 09/10/06 | 58 | 304 | 362 | 84 | <LOQ | <LOQ |
| SB | 16/10/06 | 71 | 75 | 146 | 51 | <LOQ | <LOQ |
| SB | 23/10/06 | 94 | 85 | 179 | 48 | 0.042 | <LOQ |
| WIC | 23/08/06 | <LOQ | - | 78 | - | 0.130 | 15 |
| LB | 29/09/06 | 76 | 237 | 313 | 76 | <LOD | 10 |
| LB | 05/10/06 | 418 | 669 | 1087 | 62 | <LOQ | 81 |
| LB | 10/10/06 | 307 | 915 | 1222 | 74 | <LOQ | <LOQ |
| LB | 17/10/06 | 79 | 10 | 89 | 11 | <LOD | <LOQ |
3. Experimental
3.1. Sampling activities
3.2. Mouse bioassay
3.3. Liquid chromatography-mass spectrometry
3.4. Alkaline hydrolysis
3.5. Phytoplankton analysis
4. Conclusions
Acknowledgements
References and Notes
- Draisci, R.; Lucentini, L.; Giannetti, L.; Boria, P.; Poletti, R. First report of pectenotoxin-2 (PTX-2) in algae (Dinophysis fortii) related to seafood poisoning in Europe. Toxicon 1996, 34, 923–935. [Google Scholar] [CrossRef]
- Aune, T.; Sorby, R.; Yasumoto, T.; Ramstad, H.; Landsverk, T. Comparison of oral and intraperitoneal toxicity of yessotoxin towards mice. Toxicon 2002, 40, 77–82. [Google Scholar] [CrossRef]
- Fattorusso, E.; Ciminiello, P.; Costantino, V.; Magno, S.; Mangoni, A.; Milandri, A.; Poletti, R.; Pompei, M.; Viviani, R. Okadaic acid in mussels of Adriatic Sea. Mar. Pollut. Bull. 1992, 24, 234–237. [Google Scholar] [CrossRef]
- Draisci, R.; Lucentini, L.; Giannetti, L.; Boria, P.; Stacchini, A. DetectionofdiarrhoeticshellfishtoxinsinmusselsfromItalybyionsprayliquidchromatography–massspectrometry. Toxicon 1995, 33, 1591–1603. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Magno, S.; Poletti, R.; Satake, M.; Viviani, R.; Yasumoto, T. Yessotoxin in mussels of the Northern Adriatic Sea. Toxicon 1997, 35, 177–183. [Google Scholar] [CrossRef]
- Draisci, R.; Ferretti, E.; Palleschi, L.; Marchiafava, C.; Poletti, R.; Milandri, A.; Ceredi, A.; Pompei, M. High levels of yessotoxin in mussels and presence of yessotoxin and homoyessotoxin in dinoflagellates of the Adriatic Sea. Toxicon 1999, 37, 1187–1193. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell'Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Boschetti, L.; Rubini, S.; Cangini, M.; Pigozzi, S.; Poletti, R. Complex toxin profile of Mytilus galloprovincialis from the Adriatic sea revealed by LC–MS. Toxicon 2010, 55, 280–288. [Google Scholar] [CrossRef]
- Molgó, J.; Girard, E.; Benoit, E. Cyclic Imines: An Insight into this Emerging Group of Bioactive Marine Toxins. In Phycotoxins: Chemistry and Biochemistry; Botana, L.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 319–335. [Google Scholar]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S.; Tartaglione, L.; Cangini, M.; Pompei, M.; Guerrini, F.; Boni, L.; Pistocchi, R. Toxin profile of Alexandrium ostenfeldii (Dinophyceae) from the Northern Adriatic Sea revealed by liquid chromatography–mass spectrometry. Toxicon 2006, 47, 597–604. [Google Scholar] [CrossRef]
- Marasović, I.; Ninčević, Ž.; Pavela-Vrančić, M.; Orhanović, S. A Survey of Shellfish Toxicity in the Central Adriatic Sea. J. Mar. Biol. Ass. UK 1998, 78, 1–10. [Google Scholar] [CrossRef]
- Orhanović, S.; Ninčević, Ž.; Marasović, I.; Pavela-Vrančić, M. Phytoplankton Toxins in the Central Adriatic Sea. Croat. Chem. Acta 1996, 69, 291–303. [Google Scholar]
- Pavela-Vrančić, M.; Meštrović, V.; Marasović, I.; Gillman, M.; Furey, A.; James, K.K. The occurrence of 7-epi-pectenotoxin-2 seco acid in the coastal waters of the central Adriatic (Kaštela Bay). Toxicon 2001, 39, 771–779. [Google Scholar] [CrossRef]
- Pavela-Vrančić, M.; Meštrović, V.; Marasović, I.; Gillman, M.; Furey, A.; James, K.K. DSP toxin profile in the coastal waters of the central Adriatic Sea. Toxicon 2002, 40, 1601–1607. [Google Scholar] [CrossRef]
- Ninčević Gladan, Ž.; Skejić, S.; Bužančić, M.; Marasović, I.; Arapov, J.; Ujević, I.; Bojanić, N.; Grbec, B.; Kušpilić, G.; Vidjak, O. Seasonal variability in Dinophysis spp. abundances and DSP outbreaks along the eastern Adriatic coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar]
- Vale, P.; Sampayo, M.A.M. Seasonality of diarrhetic shellfish poisoning at a coastal lagoon in Portugal: rainfull patterns and folk wisdom. Toxicon 2003, 41, 187–197. [Google Scholar] [CrossRef]
- Lassus, P.; Herbland, A.; Lebaut, C. Dinophysis blooms and toxic effects along French coast. World Aquacult. 1991, 22, 49–54. [Google Scholar]
- Klöpper, S.; Scharek, R.; Gerdts, G. Diarrhetic shellfish toxicity in relation to abundance of Dinophysis spp. in the German Bight near Helgoland. Mar. Ecol. Prog. Ser. 2003, 259, 93–102. [Google Scholar] [CrossRef]
- Koukaras, K.; Nikolaidis, G. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). J. Plankton Res. 2004, 26, 445–457. [Google Scholar] [CrossRef]
- Morano, A.; Arévalo, F.; Fernández, M.L.; Maneiro, J.; Pazos, Y.; Salgado, C.; Blanco, J. Accumulation and transformation of DSP toxins in mussels Mytilus galloprovincialis during a toxic episode caused by Dinophysis acuminata. Aquat. Toxicol. 2003, 62, 269–280. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell'Aversano, C.; Fattorusso, E.; Forino, M.; Magno, S.; Santelia, F.; Tsoukatou, M. Investigation of the toxin profile of Greek mussels Mytilus galloprovincialis by liquid chromatography—mass spectrometry. Toxicon 2006, 47, 174–181. [Google Scholar] [CrossRef]
- Lindahl, O.; Lundve, B.; Johansen, M. Toxicity of Dinophysis spp. in relation to population density and environmental conditions on the Swedish west coast. Harmful Algae 2007, 6, 218–231. [Google Scholar] [CrossRef]
- Reizopoulou, S.; Strogyloudi, E.; Giannakourou, A.; Pagou, K.; Hatzianestis, I.; Pyrgaki, C. Granéli, E. Okadaic acid accumulation in macrofilter feeders subjected to natural blooms of Dinophysis acuminata. Harmful Algae 2008, 7, 228–234. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Blanco, J. Subcellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis: First evidences of lipoprotein binding to okadaic acid. Toxicon 2010, 55, 221–226. [Google Scholar] [CrossRef]
- Rausch de Traubenberg, C.; Morlaix, M. Evidence of okadaic acid release into intracellular medium in cultures of Prorocentrum lima (Ehrenberg) Dodge. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd: Paris, France, 1995; pp. 493–498. [Google Scholar]
- Johansson, N.; Graneli, E.; Yasumoto, T.; Carlsson, P.; Legrand, C. Toxin production by Dinophysis acuminata and D. acuta cells grown under nutrient sufficient and deficient conditions. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; IOC of UNESCO: Paris, France, 1996; pp. 277–280. [Google Scholar]
- Nishitani, G.; Sugioka, H.; Imai, I. Seasonal distribution of species of the toxic dinoflagellate genus Dinophysis in Maiziru Bay (Japan), with comments on their autofluorescence and attachment of picophytoplankton. Harmful Algae 2002, 1, 253–264. [Google Scholar] [CrossRef]
- Imai, I.; Sugioka, H.; Nishitani, G.; Mitsuya, T.; Hamano, Y. Monitoring of DSP toxins in small-sized plankton fraction of seawater collected in Mutsu Bay, Japan, by ELISA method: relation with toxin contamination of scallop. Mar. Poll. Bull. 2003, 47, 114–117. [Google Scholar] [CrossRef]
- Dahl, E.; Johannessen, T. Relationship between occurrence of Dinophysis species and shellfish toxicity. Phycologia 2001, 40, 223–227. [Google Scholar]
- Svensson, S. Depuration of okadaic acid (Diarrhetic Shellfish Toxin) in mussels, Mytilus edulis (Linnaeus), feeding on different quantities of nontoxic algae. Aquaculture 2003, 218, 277–291. [Google Scholar] [CrossRef]
- Duinker, A.; Bergslien, M.; Strand, Ø.; Olseng, C.D.; Svardal, A. The effect of size and age on depuration rates of diarrhetic shellfish toxins (DST) in mussels (Mytilus edulis L.). Harmful Algae 2007, 6, 288–300. [Google Scholar] [CrossRef]
- Lindahl, O.; Hageltorn, M. Detoxification experiments of DSP in blue mussels. In. In Proceedings of the 4th Nordic Veterinary Congress, Stockholm, Sweden; 1986; pp. 463–466. [Google Scholar]
- Fernández, M.L.; Míguez, A.; Morono, A.; Cacho, E.; Martínez, A.; Blanco, J. Detoxification of low polarity toxins (DTX-3) from mussels Mytilus galloprovincialis in Spain. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 449–452. [Google Scholar]
- Blanco, J.; Fernández, M.L.; Míguez, A.; Morono, A. Okadaic acid depuration in the mussel Mytilus galloprovincialis: One- and two-compartment models and the effect of environmental conditions. Mar. Ecol. Prog. Ser. 1999, 176, 153–163. [Google Scholar] [CrossRef]
- Marcaillou-Le Baut, C.; Bardin, B.; Bardouil, M.; Bohec, M.; Le Dean, L.; Masselin, P.; Truquet, P. Toxic Phytoplankton Blooms in the Sea. In DSP Depuration Rates of Mussels Reared in a Laboratory and an Aquaculture Pond; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 531–535. [Google Scholar]
- Poletti, R.; Viviani, R.; Casadei, C.; Lucentini, L.; Funari, E.; Draisci, R. Decontamination dynamics of mussels naturally contaminated with diarrhetic toxins relocated to a basin of the Adriatic sea. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 429–432. [Google Scholar]
- Shumway, S.E.; van Egmond, H.P.; Hurst, J.W.; Bean, L.L. Manual on Harmful Marine Microalgae. In Management of Shellfish Resources; Hallegraeff, G.M., Anderson, D.M., Cembella, A., Eds.; UNESCO: Paris, France, 1995; pp. 433–462. [Google Scholar]
- Svensson, S.; Förlin, L. Analysis of the importance of lipid breakdown for elimination of okadaic acid (diarrhetic shellfish toxin) in mussels, Mytilus edulis: results from a field study and a laboratory experiment. Aquat. Toxicol. 2004, 66, 405–418. [Google Scholar] [CrossRef]
- Svensson, S. Effects, dynamics and management of okadaic acid in blue mussels, Mytilus eduls. PhD Thesis, Göteborg University, 2003; p. 50. [Google Scholar]
- Sidari, L.; Nichetto, P.; Cok, S.; Sosa, S.; Tubaro, A.; Honsell, G.; Della Loggia, R. Phytoplankton detection and DSP toxicity: methodological considerations. J. Appl. Phycol. 1995, 7, 163–166. [Google Scholar] [CrossRef]
- Marasović, I.; Skejić, S.; Bužančić, M.; Ujević, I.; Arapov, J. Harmful algal bloom monitoring program at the shell-fish breading areas through the eastern Adriatic coast (an overview) . In Book of Proceedings 2nd Congress of the Alps-Adria Working Community on Maritime, Undersea, and Hyperbaric Medicine, Zadar; Petri, N., Ed.; Naval Medical Institute of the Croatian Navy: Split, Croatia, 18–21 October 2006; pp. 145–156. [Google Scholar]
- Ujević, I.; Milandri, A.; Marasović, I.; Ceredi, A.; Pigozzi, S.; Arapov, J.; Skejić, S.; Orhanović, S.; Isajlović, I. Is Yessotoxin the Main Phycotoxin in Croatian Waters? Mari. Drugs 2010, 8, 460–470. [Google Scholar] [CrossRef]
- Ogino, H.; Kumagai, M.; Yasumoto, T. Toxicologic evaluation of yessotoxin. Nat. Toxins 1997, 5, 255–259. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Lee, J.S.; Yanagi, T.; Kenma, R.; Yasumoto, T. Fluorometric determination of diarrhetic shellfish toxins by high pressure liquid chromatography. Agric. Biol. Chem. 1987, 51, 877–881. [Google Scholar] [CrossRef]
- Scotter, M.J.; Roberts, D.P.T. Development and validation of a rapid headspace gas chromatography–mass spectrometry method for the determination of diethyl ether and acetone residues in Tween extracts of shellfish intended for mouse bioassay for diarrhoetic toxins. J. Chromatogr. A 2007, 1157, 386–390. [Google Scholar] [CrossRef]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. Spirolide composition of micro-extracted pooled cells isolated from natural plankton assemblages and from cultures of the dinoflagellate Alexandrium ostenfeldii. Nat. Toxins 1999, 7, 197–206. [Google Scholar] [CrossRef]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Stirling, D.J. Survey of historical New Zealand shellfish samples for accumulation of gymnodimine. N. Z. J. Mar. Freshwater Res. 2001, 35, 851–857. [Google Scholar] [CrossRef]
- Biré, R.; Krys, S.; Frémy, J.M.; Dragacci, S.; Stirling, D.; Kharrat, R. First evidence on occurrence of gymnodimine in clams from Tunisia. J. Nat. Toxins 2002, 11, 269–275. [Google Scholar]
- Pavela-Vrančić, M.; Ujević, I.; Furey, A. Accumulation of diarrheic toxins in the mussel Mytilus galloprovincialis from the Adriatic Sea. Croat. Chem. Acta 2006, 79, 291–297. [Google Scholar]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Matsumoto, G.L.; Clardy, J. Seafood Toxins on American Chemical Society Symposium Series. In Diarrhetic Shellfish Poisoning; Ragelis, E.P., Ed.; American Chemical Society: Washington, DC, USA, 1984; pp. 207–214. [Google Scholar]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Marcaillou-Le Baut, C.; Lucas, D.; Le Dean, L. Toxic Dinoflagellates. In Dinophysis AcuminataToxin: Status of Toxicity Bioassays in France; Anderson, D., White, A., Baden, D., Eds.; Elsevier: New York, NY, USA, 1985; pp. 485–488. [Google Scholar]
- Mountfort, D.O.; Suzuki, T.; Truman, P. Protein phosphatase inhibition assay adapted for determination of total DSP in contaminated mussels. Toxicon 2001, 39, 383–390. [Google Scholar] [CrossRef]
- Üthermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nincevic Gladan, Z.; Ujevic, I.; Milandri, A.; Marasovic, I.; Ceredi, A.; Pigozzi, S.; Arapov, J.; Skejic, S. Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006. Molecules 2011, 16, 888-899. https://doi.org/10.3390/molecules16010888
Nincevic Gladan Z, Ujevic I, Milandri A, Marasovic I, Ceredi A, Pigozzi S, Arapov J, Skejic S. Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006. Molecules. 2011; 16(1):888-899. https://doi.org/10.3390/molecules16010888
Chicago/Turabian StyleNincevic Gladan, Zivana, Ivana Ujevic, Anna Milandri, Ivona Marasovic, Alfiero Ceredi, Silvia Pigozzi, Jasna Arapov, and Sanda Skejic. 2011. "Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006" Molecules 16, no. 1: 888-899. https://doi.org/10.3390/molecules16010888
APA StyleNincevic Gladan, Z., Ujevic, I., Milandri, A., Marasovic, I., Ceredi, A., Pigozzi, S., Arapov, J., & Skejic, S. (2011). Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006. Molecules, 16(1), 888-899. https://doi.org/10.3390/molecules16010888





