Abstract
Flavonoids make up one of the most pervasive groups of plant phenolics. Due to their importance in plants and human health, it would be useful to have a better understanding of flavonoid concentration and biological activities that could indicate their potentials as therapeutic agents, and also for predicting and controlling the quality of medicinal herbs. Ginger (Zingiber officinale Roscoe) is a famous and widely used herb, especially in Asia, that contains several interesting bioactive constituents and possesses health promoting properties. In this study, total flavonoids and some flavonoid components including quercetin, rutin, catechin, epicatechin, kaempferol and naringenin were extracted from the leaves and rhizomes of two varieties of Zingiber officinale (Halia Bentong and Halia Bara) at three different growth points (8, 12 and 16 weeks after planting), and analyzed by a high performance liquid chromatography (HPLC) method in order to determine the potential of the subterranean part of the young ginger. The results showed that Halia Bara had a higher content of flavonoids in the leaves and rhizomes as compared to Halia Bentong. In both varieties, the concentration of flavonoids in the leaves decreased (Halia Bentong, 42.3%; Halia Bara 36.7%), and in the rhizomes it increased (Halia Bentong 59.6%; Halia Bara 60.1%) as the growth period increased. Quercetin was abundant in both varieties. The antioxidant activity determined by the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay showed high activities (65.7%) in the leaves of Halia Bara at 8 weeks after planting. Results suggested a good flavonoid content and antioxidant activity potential in ginger leaves at 8 weeks after planting. The leaves of these ginger varieties could be useful for both food flavourings and in traditional medicine.
1. Introduction
Plants and herbs consumed by humans may contain thousands of different phenolic acid and flavonoid components. The effect of dietary phenolics is of great current interest due to their antioxidative and possible anticarcinogenic activities [1]. Phenolic acids and flavonoids also function as reducing agents, free radical scavengers, and quenchers of singlet oxygen formation [2]. Antioxidant compounds that scavenge free radicals help protect against degenerative diseases [3]. Phenolic components play important roles in the control of cancer and other human diseases. For example, ginger has long been used in traditional medicine as a cure for some ailments including inflammatory diseases [4]. It was found that flavonoids reduce blood-lipid and glucose, and enhance human immunity [5]. Flavonoids are also a kind of natural antioxidant substances capable of scavenging free superoxide radicals, thus displaying anti-aging properties and reducing the risk of cancer. At present, flavonoids are extracted, among other sources, from ginkgo leaves [6], kudzu root [7], lotus leaves [8] and ginger rhizomes and leaves [9]. Ginger is an important horticultural crop in tropical Southeast Asia. It produces a pungent, aromatic and bioactive rhizome that is valued all over the world either as a spice or herbal medicine. Ginger is known as a resource with high phenolic contents, wide availability and low price [10], and therefore, it can serve as a cheap and important food material. Ginger is a natural food component with many active phenolic compounds such as gingerol and shagaol, and it has been shown to have anti-cancer and antioxidant effects [11]. Gingerol may reduce nausea caused by motion sickness or pregnancy and may also relieve migraines [12].
Light is known to regulate plant growth and development, and also the biosynthesis of both the primary and secondary metabolites [13,14]. Apart from their role to benefit health, antioxidants are added to foods to prevent or delay food oxidation initiated by free radicals formed during their exposure to some environmental factors such as air, light and temperature. At present most of the antioxidants in use are manufactured synthetically. In Asia, rhizomes of ginger varieties (family Zingiberaceae) have been widely used as spices in food or condiments [15]. The rhizomes are usually eaten raw or cooked as vegetables and used for flavouring foods. Traditionally, leaves of Elettariopsis latiflora (family Zingiberaceae) have been used to relieve flatulence, to improve appetite and as an antidote to poisons [16]. In Japan, leaves of Alpinia zerumbet are sold after drying as a herbal tea, and are commonly used to flavour noodles and to wrap rice cakes in celebrations. In another study the diuretic and anti-ulcerogenic properties of A. zerumbet leaves have been reported [17].
In Malaysia, only ginger rhizomes are consumed as a food flavoring and the leaves are discarded. Information on the flavonoid contents of plant foods and plant parts commonly consumed in Malaysia are still scarce. Such data would be useful to provide information on foods containing high levels of beneficial components. In the present study, we identified some of important phenolic components (flavonoids) in both of the leaves and rhizomes of two varieties of Malaysian ginger, and the antioxidant activities in these varieties was considered. The variation of flavonoid concentration as a function of growth period of young ginger was also evaluated.
2. Results and Discussion
2.1. Total flavonoids (TF)
From the data presented in Table 1, it is apparent that TF content was high in the leaves of both varieties. High levels of TF and total phenolic (TP) contents in the leaves in comparison with other parts of medicinal plants have been reported in previous studies. TF content in leaves of Psidium guajava were higher than in stems [18]. High levels of TP were reported in the leaves (291 mg/100 g fresh weight) of Zingiber officinale compared to the rhizomes (157 mg/100 g fresh weight) [4]. Leaf chloroplasts have the capability to localize phenolic compounds, some of which are specific only to these organelles [19]. It is also apparent from Table 1 that the TF contents decreased in the leaves and stems of ginger varieties from 8 to 16 weeks after planting, and vice versa, increased in the rhizomes. Comparison of the TF results between ginger (5.54–11.14 mg/g DW) and other plants, for example onion leaves (1.54 mg/g DW), semambu leaves (2.041 mg/g DW), black tea (1.41 mg/g DW), papaya (1.26 mg/g DW), bird chilli (1.66 mg/g DW), garlic (1.2 mg/g DW) and guava (1.12 mg/g DW) [20], shows the good potential of this component in these ginger varieties.

Table 1.
Accumulation and partitioning of total flavonoids in different parts of two varieties of Zingiber officinale at different harvest times (weeks after planting).
2.2. HPLC analysis results
The results obtained from the preliminary analysis of flavonoids are listed in Table 1. It was shown that Halia Bara had more flavonoids content in the leaves and rhizomes compared to Halia Bentong. What is interesting in this data is that in both varieties the concentration of flavonoids in the leaves decreased as the growth period increased, but in the rhizome it increased with increasing growth period. The decreases in Halia Bentong leaves were 10.0% (quercetin), 64.5% (rutin), 19.7% (catechin), 18.0% (epicatechin), 9.3% (naringenin) and 15.7% for kaempferol. In Halia Bara, they were 24.8% (quercetin), 2.9% (rutin), 24.4% (catechin), 42.2% (epicatechin), 2.5% (naringenin) and 21.2% for kaempferol. In the rhizome, the increases of these components in Halia Bentong were 37.5% (quercetin), 29.0% (rutin), 20.0% (catechin), 36.4% (epicatechin), 32.7% (naringenin) and 48.9% for kaempferol. In Halia Bara they were 25.5% (quercetin), 45.5% (catechin), 78.0% (epicatechin), 25.0% (naringenin) and 20.0% for kaempferol. The HPLC chromatograms from the extracts of the leaves (Figure 1) and the rhizomes (Figure 2) show some of the flavonoid compounds found in Halia Bara. The decrease of TF in Halia Bentong leaves was 42.3%, and in Halia Bara leaves it was 36.7%. The increase in TF content in Halia Bentong rhizomes was 59.6% and in Halia Bara it was 60.2%. In the family Zingiberaceae and especially Zingiber officinale, it is generally believed that secondary metabolites produced by the plants are transported to the rhizomes where they are then accumulated [21,22,23]. Najda et al. [24] have reported increases in total flavonoids in caraway roots during the vegetative growth period. This may imply that rhizomes of Z. officinale have higher stored amounts of flavonoids than other plant parts.
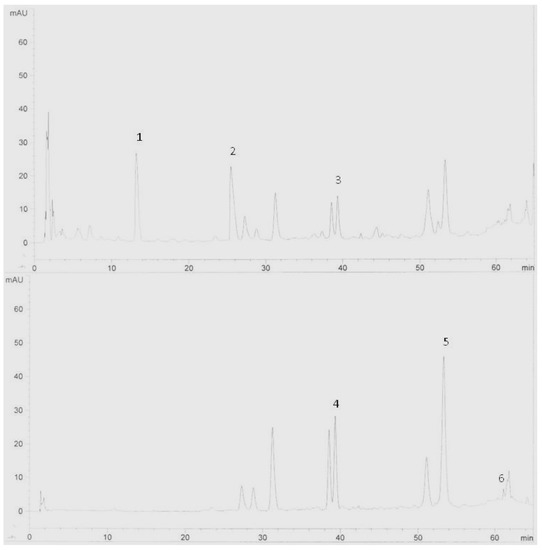
Figure 1.
HPLC chromatogram of Zingiber officinale variety Halia Bara extracts (leaves). Identification of compounds: catechin (1), epicatechin (2), naringenin (3), rutin (4), quercetin (5) and kaempferol (6).
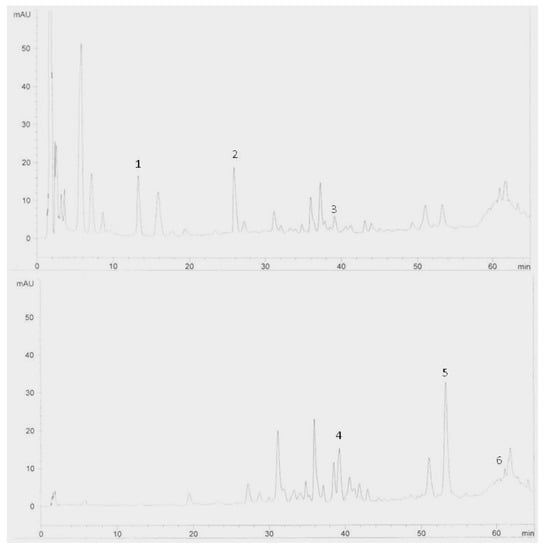
Figure 2.
HPLC chromatogram of Zingiber officinale variety Halia Bara extracts (rhizomes). Identification of compounds: catechin (1), epicatechin (2), naringenin (3), rutin (4), quercetin (5) and kaempferol (6).
Chan et al. [4] reported that leaves in ginger with high levels of TF and TP had higher antioxidant activities than rhizomes. The results of previous studies [25,26,27,28] showed that some of the flavonoid components such as quercetin and catechin had anticancer activities, and these components were able to inhibit cancer cell growth. The anticancer activities of ginger were also substantiated [29]. Results of the current research (Table 2) showed that flavonoids are important components of this plant, and that some of its pharmacological effects could be attributed to the presence of valuable TF and TP constituents. The quercetin content in the leaves and rhizomes of Halia Bara showed higher values compared to some plants, for example red chilli (0.799 mg/g DW), bird chilli (0.392 mg/g DW) and bell pepper (0.448 mg/g DW), and comparable to black tea (1.107 mg/g DW), onion (1.49 mg/g DW) and semambu (1.18 mg/g DW) [19]. A high content of quercetin (1.29 mg/g DW) was obtained in the leaves of Halia Bara at 16 weeks after planting. During the growth period variable contents of rutin and naringenin were found in the leaves and rhizomes of both varieties. Catechin and epicatechin contents in both varieties were high in the leaves at 8 weeks after planting. High content of catechin and epicatechin were detected from Halia Bara leaves (0.56 mg/ g DW; 0.19 mg/g DW) at 8 weeks after planting. Kaempferol is a rare flavonoid in plants. In Halia Bara and Halia Bentong, however, it was detected in the leaves and rhizomes in low concentrations. However, compared with green chilli (0.039 mg/g DW), sengkuang (0.037 mg/g DW), white radish (0.0383 mg/g DW) and pegaga (0.0205 mg/g DW, both ginger varieties recorded high contents (0.023–0.068 mg/g DW) of kaempferol. Ginger varieties, when compared with some plants such as cekur manis (0.323 mg/g DW), pumpkin (0.371 mg/g DW), and carrot (0.140 mg/g DW) [20], showed low contents of kaempferol. A high content of kaempferol (0.068 mg/g DW) was obtained from Halia Bara rhizomes at 16 weeks after planting.

Table 2.
Concentration of flavonoids components in the leaves and rhizomes harvested at 8, 12 and 16 weeks after planting in two varieties of Zingiber officinale.
The results of this study indicate that quercetin is the main flavonoid component in ginger. There has not been any documentation on the flavonoids components of Malaysian ginger varieties, and that our results were the first to indicate the variation of flavonoid concentration in different ginger parts during the growth period. This is an important issue for future research.
2.3. DPPH radical scavenging activity
The dose-response curve of DPPH radical scavenging activities of the methanolic extracts of the leaves and rhizomes in two varieties of Zingiber officinale at different harvest times compared with the standardized BHT and α-tocopherol are shown in Table 3 and Figure 3.

Table 3.
DPPH scavenging activities of the methanolic extracts in different parts of two varieties of Zingiber officinale. BHT and α-tocopherol were used as positive controls.
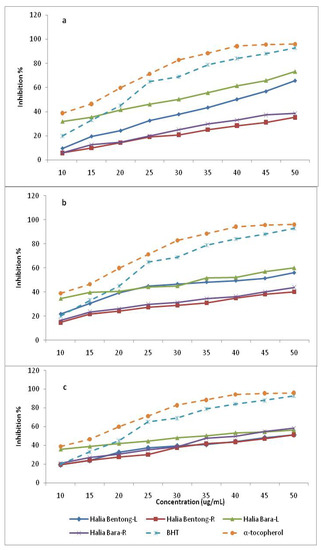
Figure 3.
DPPH radical scavenging activity of the methanolic extracts in different parts of two varieties of Zingiber officinale during the growth period compared with positive controls, BHT and α-tocopherol. L and R represent leaves and rhizomes of ginger, respectively. a, b and c signify 8, 12 and 16 weeks after planting, respectively.
It was observed that methanolic extracts of the leaves in both varieties had higher activities than those of the rhizomes at different harvest times. The free radical scavenging activities also decreased in the leaves and increased in the rhizomes from 8 to 16 weeks after planting. Leaves of Halia Bara at 8 weeks after planting had higher (73.2%) activities than those of Halia Bentong (65.7%). Similarly, the DPPH radical scavenging activity of the rhizomes of Halia Bara (58.2%) exceeded that of Halia Bentong (51.4%), showing the highest value at 16 weeks after planting. Fifty percent of the free radical scavenging observed in the leaves of both varieties at 8 weeks after planting recorded concentrations of 28 and 39 µg/mL for Halia Bara and Halia Bentong, respectively (Table 4). With increasing growth period, the IC50 in the leaves also increased (Table 4). The IC50 values of rhizome extracts were only observed at just 16 weeks after planting. A positive relationship between phenolic components and flavonoids with free radical scavenging had been reported in previous studies [9,30,31,32,33]. Since our previous results (Table 2) had shown a decrease in the concentrations of most of the flavonoids viz. quersetin, catechin and kaempferol, in the leaves from 8 to 16 weeks after planting, while they increased in the rhizomes during the same time, we could suggest that the decreasing DPPH activities of the leaves from 8 to 16 weeks could be related to the decreasing flavonoid concentrations during this growth period. A positive relationship between total flavonoids and total antioxidant activities of Zingiber officinale was also observed in this study (Figure 4).

Table 4.
IC50 values of ginger (Zingiber officinale) extracts in the DPPH assay.
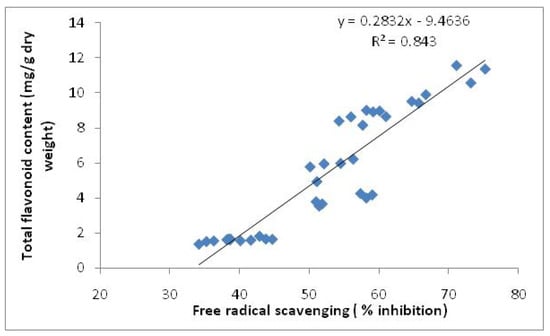
Figure 4.
Relationship between total flavonoids and total antioxidant activities of Zingiber officinale.
The increased DPPH activities of rhizomes is related to an increase and accumulation of flavonoids in the rhizomes from 8 till 16 weeks, although the DPPH radical scavenging abilities of the extracts were less than those of BHT (96.21%) and α-tocopherol (89.57%) at 45 µg/mL. The present findings seemed to be consistent with other research. Essential oils extracted from leaves of Aframomum giganteum had higher antioxidant activity compared to the rhizomes [34] whilst leaves of Alpinia zerumbet (Zingiberaceae) showed higher inhibition of β-carotene oxidation and radical scavenging activity than did rhizomes [35]. Antioxidants are secondary metabolites produced by most of plants but in different content to protect against oxidative damage by free radicals [34,35]. In the family Zingiberaceae and especially Zingiber officinale, it is generally believed that antioxidants produced by the plant are transported to the rhizomes where they are accumulated [17,36,37]. This would suggest that the rhizomes should have had higher antioxidant activity than other plant parts, but the results of this study showed that this might not be true as majority of the species studied had significantly higher flavonoids contents and antioxidant activities in the leaves than in rhizomes. Similar observations have been reported by Chan et al. [4] and Ghasemzadeh et al. [9] who observed that the leaves of ginger with high TF levels also had high antioxidant activities compared to the rhizomes. The antioxidant study showed that the ginger extracts have the good proton-donating ability and could serve as free radical inhibitors or scavengers, acting possibly as primary antioxidants.
3. Experimental
3.1. Plant material
Two varieties of Zingiber officinale Roscoe (Halia Bentong and Halia Bara) rhizome seeds were germinated in 10 cm diameter pots containing peat moss for two weeks and then transferred to 15 × 18 cm white polyethylene bags containing a soiless mixture of burnt rice husks and coco peat at a ratio of 1:1. The plants were grown under glasshouse conditions at the Faculty of Agriculture Controlled Environment Complex of University Putra Malaysia (UPM) where the daily mean irradiance was recorded at approximately 790 µmol m-2 s-1. The plants were harvested at 16 weeks, when the leaves, stems, and rhizomes were separated. Once dried (freeze dry), they were all kept at -80 ºC for future analysis.
3.2. Determination of total flavonoid contents (TF)
The TF were measured following a previously reported spectrophotometric method [38]. Briefly, extracts of each plant material (1 mL containing 0.1 mg/mL) were diluted with water (4 mL) in a 10 mL volumetric flask. Initially, 5% NaNO2 solution (0.3 mL) was added to each volumetric flask; at 5 min, 10% AlCl3 (w/w) was added; and at 6 min, 1.0 M NaOH (2 mL) was added. Water (2.4 mL) was then added to the reaction flask and mixed well. Absorbance of the reaction mixture was read at 430 nm. The results were expressed in mg quercetin/g dry weight by comparison with the quercetin standard curve, which was made in the same condition.
3.3. High Performance Liquid Chromatography (HPLC) apparatus
3.3.1. Extract preparation
Aliquots of leaves and rhizomes (0.25 g) were extracted with 60% aqueous methanol (20 mL). Then 6 M HCl (5 mL) was added to each extract to obtain a 25 mL solution of 1.2 M HCl in 50% aqueous methanol. Extracts were refluxed at 90 ºC for 2 h. Extract aliquots of 500 μL, taken both before and after hydrolysis, were filtered through a 0.45 µm filter [39].
3.3.2. Analysis of flavonoid composition by HPLC
Reversed-phase HPLC was used to assay compositions of flavonoids. The Agilent HPLC system (Tokyo, Japan) used consisted of a Model 1100 pump equipped with a multi-solvent delivery system and a L-7400 ultraviolet (UV) detector. The column type was an Agilent C18 5 µm, 4.0 mm internal diameter × 250 mm. The mobile phase composed of (A) 2% acetic acid (CH3COOH) and (B) 0.5% acetic acid-acetonitrile (CH3CN),(50:50 v/v), and gradient elution was performed as follows: 0 min, 95:5; 10 min, 90:10; 40 min, 60:40, 55 min, 45:55; 60 min, 20:80; and 65 min, 0:100. The mobile phase was filtered under vacuum through a 0.45 µm membrane filter before use. The flow rate was 1 mL/min. UV absorbance was measured at 280–365 nm. The operating temperature was maintained at room temperature [40]. Identification of the flavonoids was achieved by comparison with retention times of standards, UV spectra and calculation of UV absorbance ratios after co-injection of samples and standards. Commercial standards were purchased from Sigma–Aldrich (USA).
3.4. Determination of antioxidant activities
3.4.1. DPPH radical scavenging assay
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma–Aldrich (USA). Butylated hydroxytoluene (BHT) and α-tocopherol were purchased from Merck (India). In order to determine the radical scavenging ability, the method reported by Mensor et al. [41] was used. Briefly, 0.3 mM alcohol solution of DPPH (1 mL) was added to samples (2.5 mL) containing different concentrations originating from extracts of different parts of ginger varieties. The samples were first kept in a dark place at room temperature and their absorbance was read at 518 nm after 30 min using a spectrophotometer (U-2001, Hitachi Instruments Inc., Tokyo, Japan). The antiradical activity (AA) was determined using the following formula:
AA% = 100 − ((Abs:sample − Abs:empty sample)× 100)/ Abs:control
Empty samples containing 1 mL ethanol + 2.5 mL from various concentrations of ginger extract; control sample containing 1 mL of 0.3 mM DPPH + 2.5 mL ethanol. BHT (butylhydroxytoluene) and α-tocopherol, were used as positive controls.
3.5. Statistical analysis
The experimental design was factorial based on randomized complete block design (RCBD) and the results were expressed as mean ± standard deviation of three replicates. Where applicable, the data were subjected to one-way analysis of variance (ANOVA) and differences between factors were determined by Duncan’s Multiple Range test using the Statistical Analysis System (SAS, 1999) and MSTAT-C programme.
4. Conclusions
Flavonoids constitute an enormous collection of biologically active compounds that are ubiquitous in plants, many of which have been used in traditional Eastern medicine for thousands of years. Advances in analytical techniques such as High Performance Liquid Chromatography (HPLC) allows one to gain insight on their composition, and to study the activity of their components. Ginger has long been used for the treatment of many pathologies, so its use in the medicinal industry could be stimulated in order to develop new pharmaceuticals with particular antioxidant or antimicrobial pharmacological profiles. Herein, we investigated the properties of two Malaysian young ginger (Zingiber officinale) varieties (Halia Bentong and Halia Bara), identifying seven important flavonoid components in these two varieties and revealing for the first time the medicinal potential of their leaves.
We have also found that Halia Bara leaves and rhizomes contained more flavonoid components compared to Halia Bentong and that the leaves of both varieties can be used as food flavourings and in traditional medicine due to their good concentrations of flavonoid components. However, in order to produce young ginger rhizomes with high medicinal component quality and flavoring properties, the plant needs to be harvested at 16 weeks, although the use of the leaf flavonoids would be beneficial. Results of leaf analysis showed that the concentration of flavonoids in the leaves decreased during the growth period with subsequent flavonoid increases in the rhizomes. The levels of medicinal components in the leaves were high at 8 weeks after planting.
Acknowledgements
The authors are grateful to the Ministry of Higher Learning, Malaysia for financing this work under the Fundamental Research Grant Scheme FRGS/PHASE1-2009/FUNDAMENTAL SCIENCE/UPM/ (01-11-08-646FR).
References
- Malencic, D.; Popovic, M.; Miladinovic, J. Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr. seeds. Molecules 2007, 12, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Andlauer, W.; Fürst, P. Antioxidative power of phytochemicals with special reference to cereals. Cereal Food. World 1998, 43, 356–360. [Google Scholar]
- Amin, I.; Tan, S.H. Antioxidant activity of selected commercial seaweeds. Malaysian J. Nutr. 2002, 8, 167–177. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Atoui, K.; Mansouri, A.; Bosku, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Feng, X.P.; Cha, Z.Y.; Wang, H.S. Study on the extractive technology of total flavones from foliage of Gingko. Chem. Ind. Corros. Control Sichuan 2002, 138. [Google Scholar]
- Liao, H.B.; He, Z.E. Research and prospect of kudzu root. Natl. Food Assoc. 2003, 24, 81–83. [Google Scholar]
- Chen, H.G.; Yu, Y.G.; Zeng, O.X. Study on extraction of flavonoids and alkaloids from lotus leaf. Food Sci. 2002, 23, 69–71. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Rozanida, A.R.; Nurul Izza, N.; Mohd Helme, M.H.; Zanariah, H. Cosmaceutical Product from Species in the Family Zingiberaceae. In Harnessing Cures from Nature: Trends and Prospects; Mazura, M.P., Ed.; Forest Research Institute: Kepong, Selangor, Malaysia, 2006; pp. 31–36. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemical. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, T. Ginger (Zingiber officinale) in migraine headache. J. Ethnopharmacol. 1990, 29, 267–273. [Google Scholar] [CrossRef]
- Liu, C.Z.; Guo, C.; Wang, Y.C.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua. Process Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Jaafar, H.Z.E.; Mohamed Haris, N.B.; Rahmat, A. Accumulation and partitioning of total phenols in two varieties of Labisia pumila Benth under manipulation of greenhouse irradiance. Acta Hort. 2008, 797, 387–392. [Google Scholar] [CrossRef]
- Larsen, K.; Ibrahim, H.; Khaw, S.H.; Saw, L.G. Gingers of Peninsular Malaysia and Singapore. Kota Kinabalu:. Nat. Hist. Publ. (Borneo) 1999, 135. [Google Scholar]
- Ravindran, P.N.; Nirmal Babu, K. Ginger the genus Zingiber. Med. Aromat. Plants-Ind. Profiles 2005, 545. [Google Scholar]
- Mpalantinos, M.A.; De Moura, R.S.; Parente, J.P.; Kuster, R.M. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Pongsak, R.; Parichat, P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J. Med. Plants Res. 2010, 4, 393–396. [Google Scholar]
- Valentine, I.K.; Maria, V.K; Bruno, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Khoo, H.M.; Suhaila, M. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay and Folin–Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Mpalantinos, M.A.; De Moura, R.S.; Parente, J.P.; Kuster, R.M. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Habsah, M.; Amran, M.; Mackeen, M.M.; Lajis, N.H. Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J. Ethnopharmacol. 2000, 72, 403–410. [Google Scholar] [CrossRef]
- Najda, A.; Dyduch, J.; Brzozowski, N. Flavonoid content and antioxidant activity of Caraway roots (Carum carvil.). Veg. Crops Res. Bull. 2008, 68, 127–133. [Google Scholar]
- Elattar, T.M.; Virji, A.S. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000, 20, 1733–1738. [Google Scholar] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G. Quercetin inhibits p21-ras expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 1999, 85, 438–445. [Google Scholar] [CrossRef]
- Arts, I.C.; Jacobs, D.R.J.; Gross, M.; Harnack, L.J.; Folsom, A.R. Dietary catechins and cancer incidence among postmenopausal women: The Iowa Women’s Health Study (United States). Cancer Cause Control 2002, 13, 373–382. [Google Scholar] [CrossRef]
- Shukla, Y.; Prasad, S.; Tripathi, C.; Singh, M.; George, J.; Kalra, N. In vitro and in vivo modulation of testosterone mediated alterations in apoptosis related proteins by [6]-gingerol. Mol. Nutr. Food Res. 2007, 51, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Pongsak, R.; Parichat, P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J. Med. Plants Res. 2010, 4, 393–396. [Google Scholar]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Hasna, O.; Afidah, A. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecules 2009, 14, 970–978. [Google Scholar]
- Praven, K.; Ramamoorty, A.; Awang, B. Anti oxidant activity, total phenolic and flavonoid content Morinda citrifolia fruit. J. Eng. Sci. 2007, 2, 70–80. [Google Scholar]
- Agnaniet, H.; Menut, C.; Bessiere, J.M. Aromatic plants of tropical central Africa. Part XLIX: Chemical composition of essential oils of the leaf and rhizomes of Aframomum giganteum K. Schum from Gabon. Flavor Frag. J. 2004, 19, 205–209. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Essential oils, kava pyrones and phenolic compounds from leavess and rhizomess of Alpinia zerumbet and their antioxidant activity. Food Chem. 2007, 103, 486–494. [Google Scholar] [CrossRef]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Zaeoung, S.; Plubrukarn, A.; Keawpradub, N. Cytotoxic and free radical scavenging activities of Zingiberaceous rhizomes. Songklanakarin J. Sci. Technol. 2005, 27, 799–812. [Google Scholar]
- Bushra, S.; Farooq, A.; Muhammad, A. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar]
- Crozier, A.; Jensen, E.; Lean, M.E.J.; Mc Donald, M.S. Quantitative analysis of flavonoids by reversed-phase high performance liquid chromatography. J. Chromatogr. 1997, 761, 315–321. [Google Scholar] [CrossRef]
- Wang, T.C.; Chuang, Y.C.; Ku, Y.H. Quantification of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; Santos, T.S.; Coube, C.S.I. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).