Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids
Abstract
:1. Introduction
2. Results and Discussion


2.1. Thin Layer Chromatographic (TLC) profile modified with DPPH method

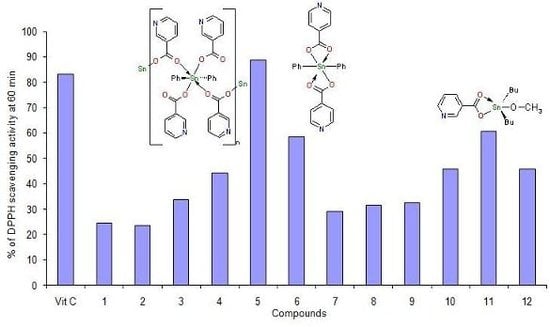
2.2.DPPH radical scavenging activity



2.3. Ferric-reducing antioxidant power assay (FRAP)


3. Experimental
3.1. General
3.2. Synthesis
3.3. Thin Layer Chromatographic (TLC) profile modified with DPPH method
3.4. Antiradical activity measurement with DPPH
3.5. Ferric-reducing antioxidant power assay (FRAP)
4. Conclusions
Acknowledgments
References
- Berners, S.J. Metals in medicine. Keynote Lectures. KL01:The mitochondrial cell death pathway as a target for gold and other metal-based antitumor compounds. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Farrell, N. Metals in medicine. Keynote Lectures. KL02: Transplatinum compounds as anticancer agents. A real posibility? J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Hambley, T.W. Metals in medicine. Keynote Lectures. KL03:The distribution and biotransformation of metal based anticancer agents in cancer cells and tumor models. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Orving, C. Metals in medicine. Multifunctional carbohydrate-appended metal complexes as potential agents in alzheimer's disease therapy. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Crichton, R. Metals in medicine. Keynote lectures. SL006: Iron generated reactive oxygen species and neurodegenerative diseases. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Clarke, M.J. Metals in medicine. Keynote lectures. SL005: Overview of non-platinum therapeutic and diagnostic agents. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Mendes, I.C.; Moreira, J.P.; Ardisson, J.D.; Santos, R.G.D.; Silva, P.R.O.D.; García, I.; Castiñeiras, A.; Beraldo, H. Organotin(IV) complexes of 2-pyridineformamide-derived thiosemicarbazones: Antimicrobial and cytotoxic effects. Eur. J. Med. Chem. 2007, 1–8. [Google Scholar]
- Tripathi, U.N.; Ahmad, M.S.; Venubabu, G.; Ramakrishna, P. Synthesis, spectral and antimicrobial studies of triorganotin(IV) 3(2'-hydroxyphenyl)-5-(4-substituted phenyl) pyrazolinates. J. Coord. Chem. 2007, 1–12. [Google Scholar]
- Bernhardt, P. Metals in medicine. Keynote lectures. SL003: Chelators for the treatment of iron overload diseases. J. Biol. Inorg. Chem. 2007, 12, S7–S52. [Google Scholar] [CrossRef]
- Jiménez-Pérez, V.M.; Camacho-Camacho, C.; Ramos-Organillo, A.; Ramirez-Trejo, R.; Peña-Hueso, A.; Contreras, R.; Flores-Parra, A. Hypervalent and binuclear silicon and germanium derivatives from bis-(3,5-di-tert-butyl-2-phenol)-oxamide. J. Organomet. Chem. 2007, 692, 5549–5554. [Google Scholar] [CrossRef]
- Peña-Hueso, A.; Esparza-Ruiz, A.; Ramos-García, I.; Flores-Parra, A.; Contreras, R. Triphenyl lead, tin and germanium coordination compounds derived from 9H-3-thia-1, 4a, 9-triaza-fluorene-2,4-dithione. J. Organomet. Chem. 2008, 693, 492–504. [Google Scholar]
- Gleeson, B.; Claffey, J.; Ertler, D.; Hogan, M.; Müller-Bunz, H.; Paradisi, F.; Wallis, D.; Tacke, M. Novel organotin antibacterial and anticancer drugs. Polyhedron 2008, 27, 3619–3624. [Google Scholar] [CrossRef]
- Mendes, I.C.; Moreira, J.P.; Ardisson, J.D.; dos Santos, R.G.; da Silva, P.R.O.; Garcia, I.; Castineiras, A.; Beraldo, H. Organotin(IV) complexes of 2-pyridineformamide-derived thiosemicarbazones: Antimicrobial and cytotoxic effects. Eur. J. Med. Chem. 2008, 43, 1454–1461. [Google Scholar] [CrossRef]
- Katsoulakou, E.; Tiliakos, M.; Papaefstathiou, G.; Terzis, A.; Raptopoulou, C.; Geromichalos, G.; Papazisis, K.; Papi, R.; Pantazaki, A.; Kyriakidis, D.; Cordopatis, P.; Manessi-Zoupa, E. Diorganotin(IV) complexes of dipeptides containing the alpha-aminoisobutyryl residue (Aib): Preparation, structural characterization, antibacterial and antiproliferative activities of [(n-Bu)(2)Sn(H-L-1)] (LH = H-Aib-L-Leu-OH, H-Aib-L-Ala-OH). J. Inorg. Biochem. 2008, 102, 1397–1405. [Google Scholar] [CrossRef]
- Nath, M.; Sulaxna; Song, X.; Eng, G.; Kumar, A. Synthesis and spectral studies of organotin(IV) 4-amino-3-alkyl-1,2,4-triazonle-5-thionates: In vitro antimicrobial activity. Spectrochim. Acta Part A 2008, 70, 766–774. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, P.; Kumar, V.; Hashmi, A.A. Organotin(IV) oxo-homoscorpionate: preparation, spectroscopic characterization and antimicrobial properties. J. Coord. Chem. 2008, 61, 1283–1293. [Google Scholar] [CrossRef]
- Balas, V.I.; Hadjikakou, S.K.; Hadjiliadis, N.; Kourkoumelis, N.; Light, M.E.; Hursthouse, M.; Metsios, A.K.; Karkabounas, S. Crystal structure and antitumor activity of the novel zwitterionic complex of tri-n-Butyltin(IV) with 2-thiobarbituric acid. Bioinorg. Chem. Appl. 2008, 2008, 1–5. [Google Scholar]
- Kovala-Demertzi, D.; Dokorou, V.; Primikiri, A.; Vargas, R.; Silvestru, C.; Russo, U.; Demertzis, M.A. Organotin meclofenamic complexes: Synthesis, crystal structures and antiproliferative activity of the first complexes of meclofenamic acid - Novel anti-tuberculosis agents. J. Inorg. Biochem. 2009, 103, 738–744. [Google Scholar] [CrossRef]
- Hadjikakou, S.K.; Hadjiliadis, N. Antiproliferative and anti-tumor activity of organotin compounds. Coord. Chem. Rev. 2009, 253, 235–249. [Google Scholar] [CrossRef]
- Nath, M.; Jairath, R.; Eng, G.; Song, X.Q.; Kumar, A. Synthesis, spectral characterization and biological studies of some organotin(IV) complexes of L-proline, trans-hydroxy-L-proline and L-glutamine. Spectrochim. Acta Part a-Mol. Biomol. Spec. 2005, 62, 1179–1187. [Google Scholar] [CrossRef]
- Nath, M.; Pokharia, S.; Eng, G.; Song, X.Q.; Kumar, A. New triorganotin (IV) derivatives of dipeptides as models for metal-protein interactions: Synthesis, structural characterization and biological studies. Spectrochim. Acta Part a-Mol. Biomol. Spec. 2006, 63, 66–75. [Google Scholar] [CrossRef]
- Rauf, M.K.; Saeed, M.A.; Imtiaz ud, D.; Bolte, M.; Badshah, A.; Mirza, B. Synthesis, characterization and biological activities of some new organotin(IV) derivatives: Crystal structure of [(Sn Ph-3) (OOCC6H4OH)] and [(SnMe3)(2) (OOC)(2)C6Cl4 (DMSO)(2)]. J. Organomet. Chem. 2008, 693, 3043–3048. [Google Scholar] [CrossRef]
- Gonzalez, A.; Gomez, E.; Cortes-Lozada, A.; Hernandez, S.; Ramirez-Apan, T.; Nieto-Camacho, A. Heptacoordinate Tin(IV) Compounds Derived from Pyridine Schiff Bases: Synthesis, Characterization, in Vitro Cytotoxicity, Anti-inflammatory and Antioxidant Activity. Chem. Pharm. Bull. 2009, 57, 5–15. [Google Scholar] [CrossRef]
- Carrera, N.; Gutiérrez, E.; Benavente, R.; Villavieja, M.M.; Albéniz, A.C.; Espinet, P. Stannylated Polynorbornenes as New Reagents for a Clean Stille Reaction. Chem. Eur. J. 2008, 14, 10141–10148. [Google Scholar]
- Dokorou, V.N.; Kovala-Demertzi, D.; Louloudi, M.; Silvestru, A.; Demertzis, M.A. Synthesis, characterization and catalytic properties of diorganotin derivatives. Crystal and molecular structure of the first complex of 2-(2-methyl-3-nitroanilino)benzoic acid of 1,2:3,4-di-l2-2-(2-methyl-3-nitroanilino)benzoato-O,O-1,3-bis-2-(2-methyl-3-nitroanilino)-benzoato-O-1,2,4:2,3,4-di-l3-oxo-tetrakis[di-methyltin(IV)]. J. Organomet. Chem. 2008, 693, 3587–3592. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta Part a-Mol. Biomol. Spec. 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant property of quercetin-Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Tahir, M.M.; Bhanger, M.I. Synthesis, characterization and investigation of antioxidant activity of cobalt-quercetin complex. J. Mol. Struct. 2008, 892, 39–46. [Google Scholar] [CrossRef]
- Gabrielska, J.; Soczynska-Kordala, M.; Hladyszowski, J.; Zylka, R.; Miskiewicz, J.; Przestalski, S. Antioxidative effect of quercetin and its equimolar mixture with phenyltin compounds on liposome membranes. J. Agric. Food Chem. 2006, 54, 7735–7746. [Google Scholar]
- Gabrielska, J.; Monika, S.-K.; Prestalski, S. Antioxidative effecto of kempferol and its equimolar mixture with phenyltin compounds on UV-irradiated liposome membranes. J. Agric. Food Chem. 2005, 53, 76–83. [Google Scholar]
- Bardak, F.; Atac, A.; Kurt, M. Infrared and Raman study of some isonicotinic acid metal(II) halide adn tetracyanonickelate complexes. Spectrochim. Acta A 2009, 71, 1896–1900. [Google Scholar] [CrossRef]
- Carlson, L.A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 2005, 258, 94–114. [Google Scholar]
- Kalinowska, M.; Borawska, M.; Swislocka, R.; Piekut, J.; Lewandowski, W. Spectroscopic (IR, Raman, UV, 1H and 13C NMR) and microbiological studies of Fe(II), Ni(II), Cu (II), Zn(II) and Ag(I) picolinates. Mol. Struct. 2007, 834, 419–425. [Google Scholar] [CrossRef]
- Morton, I.K.M.; Hall, J.M.P. Nicotinic Acid. In Concise Dictionary of Pharmacological Agents - Properties and Synonyms, 1st ed; Springer-Verlag: London, UK, 1999. [Google Scholar]
- Basu, T.K.; Mann, S. Vitamin B6 Normalizes the Altered Sulfur Amino Acid Status of Rats Fed Diets containing Pharmacological Levels of Niacin without Reducing Niacin’s Hypolipidemic Effects. J. Nutr. 1997, 127, 117–121. [Google Scholar]
- Szorcsik, A.; Nagy, L.; Scopelliti, M.; Deak, A.; Pellerito, L.; Galbács, G.; Hered, M. Preparation and structural characterization of [Ph3Sn(IV)]+ complexes with pyridine-carboxylic acids of hydroxypyridine, -pyrimidine and quinoline. J. Organomet. Chem. 2006, 691, 1622–1630. [Google Scholar] [CrossRef]
- Theo, S.-G.; Ang, S.-H.; Lim, H.-C.; Fun, H.-K.; Ibrahim, A.R. Syhtesis and Crystal Structure of Polymeric Triphenyltin(IV) 4-pyridinecarboxylate. J. Coord. Chem. 1998, 46, 87–96. [Google Scholar] [CrossRef]
- Win, Y.F.; Guan, T.S.; Yamin, B.M. Synthesis and characterization of tributyltin(IV) complexes derived from pyridine monocarboxylic acids. The Malaysian J. Anal. Sci. 2006, 10, 285–294. [Google Scholar]
- Corona-Bustamante, A. Sintesis, caracterización y evaluación farmacológica de estanoxanos derivados de los ácidos [2-], [3-] y 4-piridín carboxilicos. Doctoral Thesis, Universidad de Colima, Coquimatlán, Colima, 2009. [Google Scholar]
- Gielen, M.; Dalil, H.; Ghys, L.; Boduszek, B.; Tienkink, E.R.T.; Martins, J.C.; Biesemans, M.; Willem, R. Synthesis and structure of di-n-butyltin pyridine-2-phosphonate-6-carboxylate. Organometallics 1998, 17, 4259–4262. [Google Scholar]
- Bafna, A.; Mishra, S. Actividad antioxidante in vitro del extracto de metanol de los rizomas de Curculigo orchioides Gaertn. Ars. Pharm. 2005, 46, 125–138. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Pérez, R.; Vargas, R.; Martínez, F.; García, E.; Hernández, B. Antioxidant activity of alkaloids from Bocconia arborea. A study on six testing methods. Ars. Pharm. 2003, 44, 5–21. [Google Scholar]
- Bukhari, S.B.; Memon, S.; Tahir, M.M.; Bhanger, M.I. Synthesis, characterization and investigation of antioxidant activity of cobalt-quercetin complex. J. Mol. Struct. 2008, 892, 39–46. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant property of quercetin-Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Gabrielska, J.; Sczynska, M.; Prezestalski, S. Antioxidative effect of kaempferol and its equimolar mixture with phenyltin compounds on UV-irradiated liposome membranes. J. Agric. Food Chem. 2005, 53, 76–83. [Google Scholar] [CrossRef]
- Carlson, L.A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Int. Med. 2005, 258, 94–114. [Google Scholar] [CrossRef]
- Bardak, F.; Atac, A.; Kurt, M. Infrared and Raman study of some isonicotinic acid metal (II) halide and tetracyanonickelate complexes. Spectrochim. Acta Part A 2009, 71, 1896–1900. [Google Scholar] [CrossRef]
- Murakami, K.; Tanemura, Y.; Yoshino, M. Dipicolinic acid prevents the copper-dependent oxidation of low density lipoprotein. J. Nutr. Biochem. 2003, 14, 99–103. [Google Scholar] [CrossRef]
- Topçu, G.; Ertaş, A.; Kolak, U.; Öztürk, M.; Ulubelen, A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. Arkivoc 2007, vii, 195–208. [Google Scholar]
- Appel, K.E. Organotin Compounds: Toxicokinetic Aspects. Drug Metab. Rev. 2004, 36, 763–786. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR nomenclature: nuclear spin properties and conventions for chemical shifts. IUPAC Recommendations 2001. Magn. Reson. Chem. 2002, 40, 489. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F. Antioxidant and cytotoxic activities of methanolic extract and fractions from Senecio gibbosus subsp. gibbosus (GUSS) DC. Nat. Prod. Res. 2006, 20, 805–812. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Corona-Bustamante, A.; Viveros-Paredes, J.M.; Flores-Parra, A.; Peraza-Campos, A.L.; Martínez-Martínez, F.J.; Sumaya-Martínez, M.T.; Ramos-Organillo, Á. Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids. Molecules 2010, 15, 5445-5459. https://doi.org/10.3390/molecules15085445
Corona-Bustamante A, Viveros-Paredes JM, Flores-Parra A, Peraza-Campos AL, Martínez-Martínez FJ, Sumaya-Martínez MT, Ramos-Organillo Á. Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids. Molecules. 2010; 15(8):5445-5459. https://doi.org/10.3390/molecules15085445
Chicago/Turabian StyleCorona-Bustamante, Alicia, Juan Manuel Viveros-Paredes, Angelina Flores-Parra, Ana Lilia Peraza-Campos, Francisco J. Martínez-Martínez, María Teresa Sumaya-Martínez, and Ángel Ramos-Organillo. 2010. "Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids" Molecules 15, no. 8: 5445-5459. https://doi.org/10.3390/molecules15085445