Changes of Flavan-3-ols with Different Degrees of Polymerization in Seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ Grapes after Veraison
Abstract
:1. Introduction
2. Results and Discussion
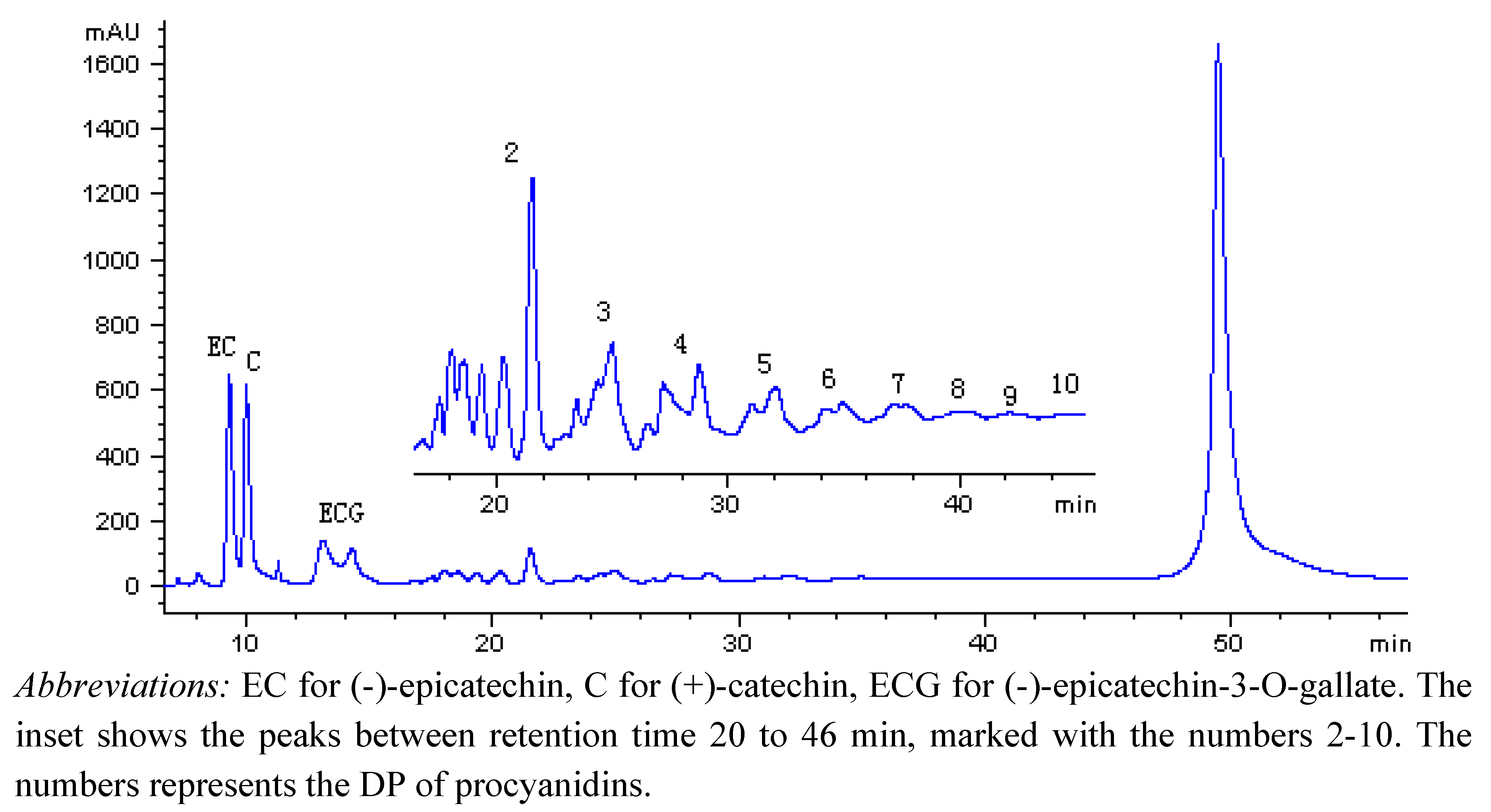
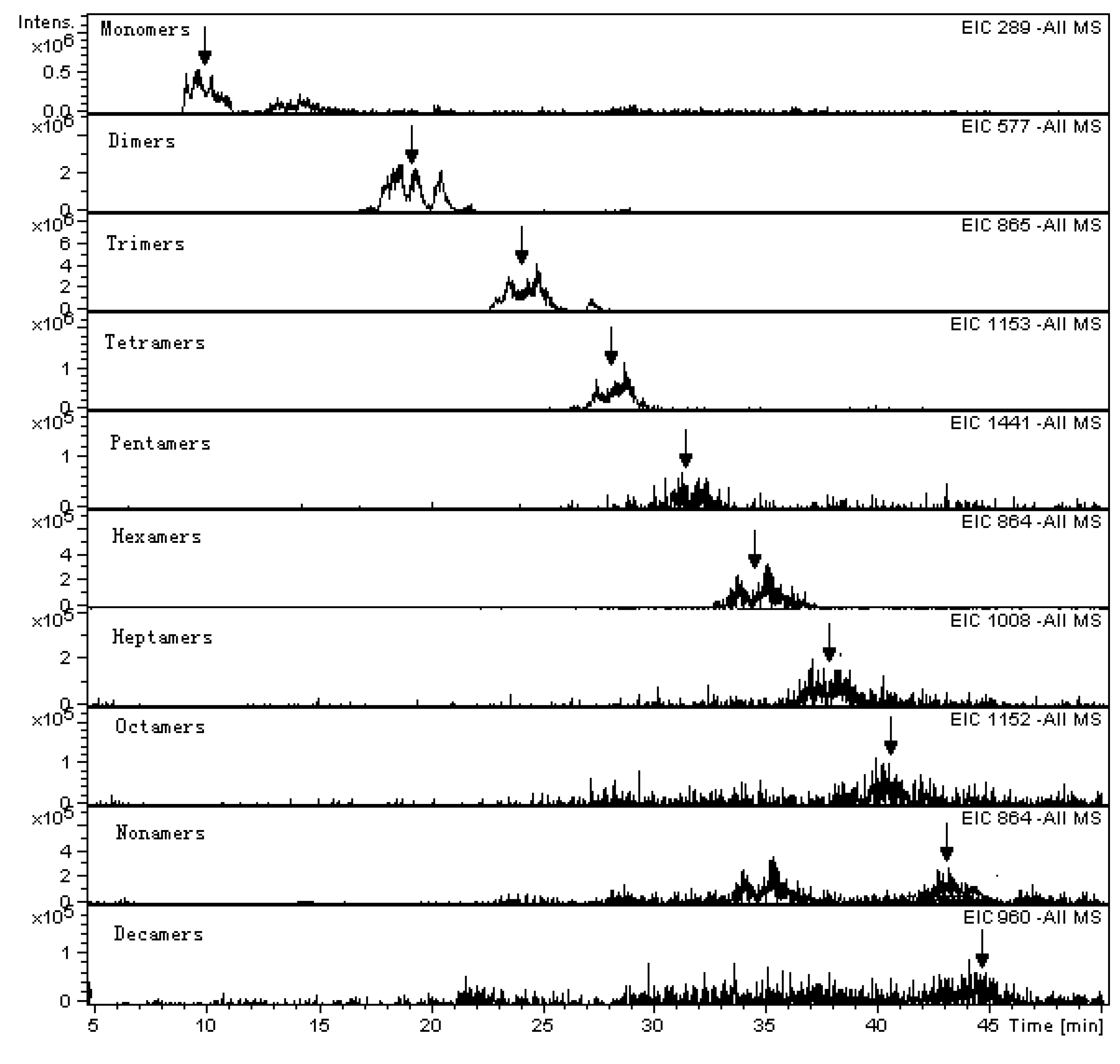
| Retention time (min) (min) | Degree of Polymerization | m/z of ions | |||
|---|---|---|---|---|---|
| [M-H]- | [M-2H]2-/2 | [M-3H]3-/3 | |||
| 9.9 | 1 | 289 | |||
| 20.0 | 2 | 577 | |||
| 25.0 | 3 | 865 | |||
| 29.0 | 4 | 1153 | 576 | ||
| 32.0 | 5 | 1441 | 720 | ||
| 34.0 | 6 | 1729 | 864 | ||
| 38.1 | 7 | 1008 | 672 | ||
| 41.2 | 8 | 1152 | 768 | ||
| 43.0 | 9 | 1296 | 864 | ||
| 45.0 | 10 | 1441 | 960 | ||
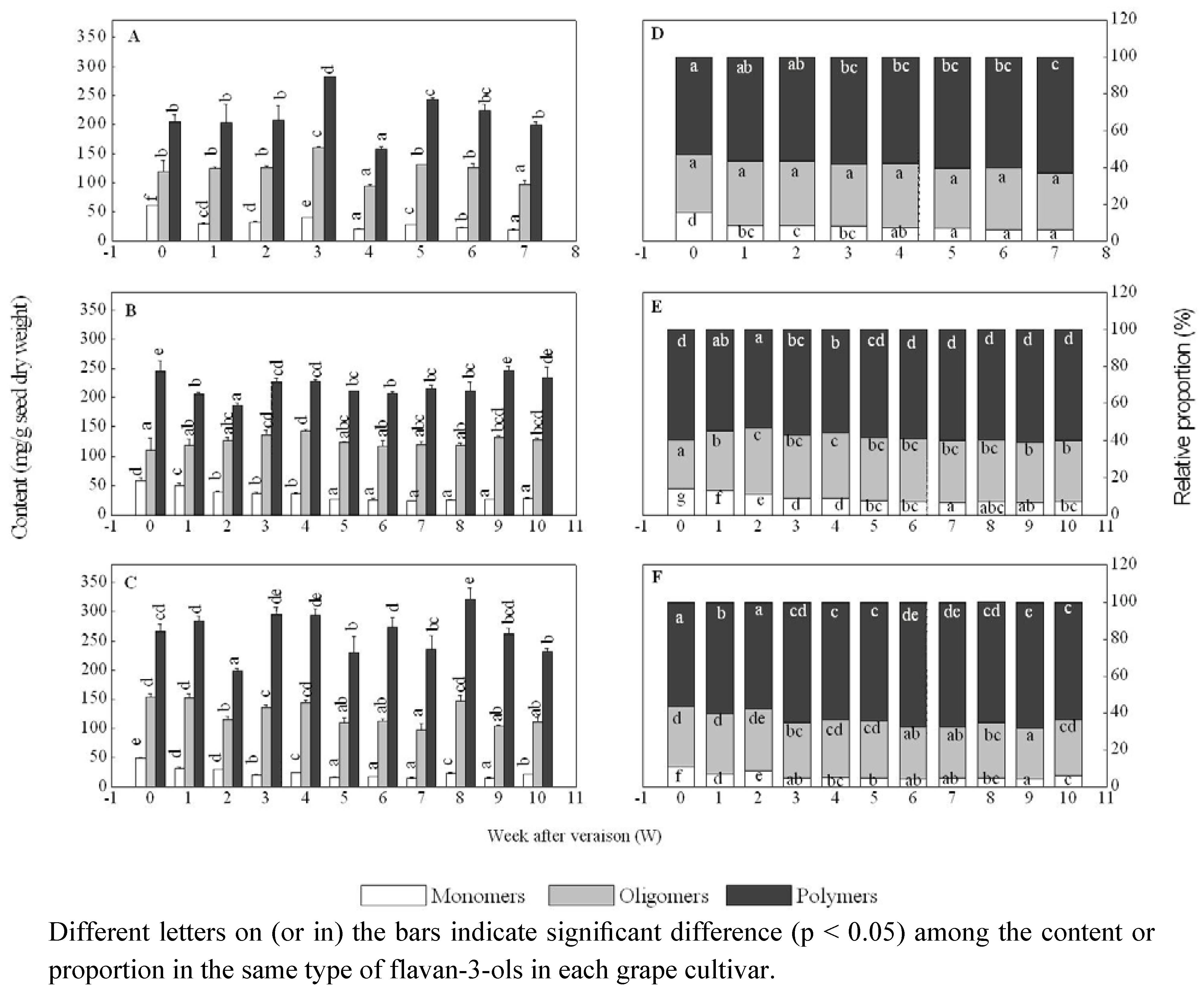
2.1. Changes in monomeric flavan-3-ols after veraison

2.2. Changes in oligomeric flavan-3-ols after veraison
| Grape cultivar | Weeks after veraison | Content (mg/g seed DW) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2 | P3 | P4 | P5 | P6 | P7 | P8-10 | ||||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |||
| 0 | 26.56b | 3.43 | 14.15b | 1.90 | 14.69b | 2.02 | 11.16ab | 2.01 | 10.24ab | 2.12 | 9.97abc | 2.58 | 33.29b | 5.21 | ||
| 1 | 25.75b | 0.77 | 14.73b | 0.27 | 15.89bc | 0.57 | 12.80ab | 1.25 | 10.94bc | 0.35 | 11.23bc | 1.18 | 33.69b | 1.45 | ||
| 2 | 26.15b | 0.85 | 14.82b | 0.23 | 16.14bc | 0.64 | 13.42bc | 1.03 | 11.19bc | 0.38 | 10.75abc | 0.35 | 34.21b | 1.03 | ||
| ‘Shiraz’ | 3 | 32.80c | 0.17 | 19.27c | 1.15 | 21.22d | 0.13 | 17.36d | 0.12 | 14.74d | 0.25 | 13.93d | 0.97 | 41.46c | 1.29 | |
| 4 | 18.21a | 0.73 | 11.69a | 0.19 | 12.32a | 1.04 | 10.34a | 0.82 | 8.93a | 0.41 | 9.34ab | 0.34 | 23.79a | 2.19 | ||
| 5 | 25.11b | 0.98 | 15.90b | 0.41 | 17.64c | 0.67 | 14.33c | 0.91 | 12.22c | 0.33 | 12.19cd | 1.01 | 34.20b | 3.56 | ||
| 6 | 23.58b | 1.35 | 14.92b | 1.12 | 16.08bc | 0.49 | 13.60bc | 0.95 | 11.71bc | 0.96 | 11.28bc | 0.48 | 35.39b | 1.05 | ||
| 7 | 19.13a | 1.27 | 11.74a | 1.06 | 12.57a | 0.71 | 10.56a | 1.19 | 8.88a | 0.76 | 8.80a | 0.30 | 26.08a | 1.91 | ||
| 0 | 22.71a | 4.13 | 13.48a | 2.65 | 14.30a | 2.01 | 11.14a | 2.15 | 10.49a | 2.52 | 9.91a | 1.82 | 27.91a | 7.17 | ||
| 1 | 24.20a | 1.28 | 13.81a | 0.80 | 15.51ab | 0.79 | 12.59a | 1.58 | 11.79ab | 1.77 | 11.15ab | 2.22 | 30.49ab | 3.94 | ||
| 2 | 25.22ab | 0.53 | 15.90ab | 0.34 | 16.63ab | 0.47 | 13.28ab | 0.76 | 12.03ab | 1.67 | 11.98ab | 1.28 | 31.57ab | 1.95 | ||
| 3 | 25.21ab | 1.78 | 16.56ab | 1.46 | 17.61b | 1.25 | 14.74ab | 1.05 | 13.00ab | 1.08 | 12.59b | 1.23 | 37.34b | 1.03 | ||
| ‘Cabernet Sauvignon’ | ||||||||||||||||
| 4 | 26.38b | 0.10 | 17.92b | 0.69 | 18.70b | 0.42 | 15.46b | 0.41 | 13.95b | 0.41 | 14.04b | 0.60 | 37.66b | 0.66 | ||
| 5 | 22.82a | 0.59 | 15.54ab | 0.47 | 15.79ab | 0.45 | 13.60a | 0.92 | 12.01ab | 0.22 | 11.76ab | 0.18 | 32.47ab | 0.49 | ||
| 6 | 21.45a | 1.73 | 14.26ab | 1.12 | 15.22ab | 1.53 | 12.86a | 1.04 | 11.90ab | 1.50 | 11.51ab | 0.69 | 30.86ab | 1.38 | ||
| 7 | 21.27a | 1.10 | 14.08ab | 0.27 | 15.15ab | 0.09 | 12.64a | 0.71 | 12.05ab | 0.70 | 11.32ab | 0.19 | 33.76ab | 3.00 | ||
| 8 | 21.59a | 0.69 | 14.31ab | 0.46 | 15.36ab | 0.76 | 12.98a | 0.47 | 12.77ab | 0.21 | 11.29ab | 0.85 | 30.30ab | 1.99 | ||
| 9 | 22.26a | 0.81 | 15.61ab | 0.23 | 16.80ab | 0.30 | 14.29b | 0.04 | 13.24b | 0.60 | 12.66b | 0.49 | 38.20b | 0.37 | ||
| 10 | 22.93a | 0.32 | 15.97ab | 0.57 | 16.74ab | 0.67 | 13.67ab | 0.76 | 13.02ab | 0.56 | 12.31b | 0.51 | 33.47b | 1.75 | ||
| 0 | 28.99b | 1.31 | 19.42b | 0.77 | 17.66b | 1.06 | 15.86ab | 0.67 | 14.20b | 1.02 | 13.42b | 0.38 | 44.01b | 2.76 | ||
| 1 | 24.48ab | 3.14 | 17.63ab | 1.38 | 17.07b | 0.93 | 15.95ab | 1.01 | 15.15b | 1.27 | 14.10b | 0.29 | 47.37b | 0.74 | ||
| 2 | 20.78a | 0.96 | 13.45a | 0.38 | 14.20a | 0.25 | 12.03a | 0.71 | 11.03a | 0.62 | 10.49a | 0.70 | 33.08a | 2.56 | ||
| 3 | 21.25ab | 0.46 | 16.20ab | 0.05 | 15.64ab | 0.83 | 14.10a | 0.35 | 14.20b | 0.66 | 13.07b | 1.03 | 40.83ab | 4.21 | ||
| ‘Marselan’ | 4 | 23.53b | 0.29 | 17.53ab | 0.24 | 17.34b | 0.20 | 15.49cd | 0.13 | 15.11b | 0.18 | 13.58b | 0.46 | 41.75ab | 3.28 | |
| 5 | 16.75a | 0.94 | 12.70a | 0.50 | 12.91a | 0.57 | 11.83a | 0.90 | 11.12a | 1.12 | 10.63a | 0.70 | 34.17a | 3.17 | ||
| 6 | 18.14a | 1.01 | 13.57a | 0.72 | 13.59a | 0.67 | 12.78a | 0.98 | 11.36a | 0.51 | 10.51a | 0.41 | 32.81a | 0.99 | ||
| 7 | 16.22a | 1.69 | 11.97a | 1.37 | 11.30a | 1.29 | 11.10a | 1.22 | 9.47a | 1.07 | 9.14a | 0.56 | 28.57a | 2.56 | ||
| 8 | 23.77ab | 1.35 | 17.95ab | 1.28 | 17.19b | 1.28 | 16.96b | 1.24 | 13.86b | 2.13 | 13.71b | 0.56 | 42.62ab | 2.37 | ||
| 9 | 17.56a | 0.24 | 12.56a | 0.09 | 11.88a | 0.50 | 11.62a | 0.63 | 10.36a | 0.44 | 9.94a | 0.11 | 30.71a | 0.20 | ||
| 10 | 18.40a | 0.61 | 12.35a | 0.36 | 13.74a | 0.68 | 11.53a | 1.48 | 10.94a | 0.70 | 10.68a | 1.47 | 33.25a | 3.23 | ||
2.3. Changes in polymeric flavan-3-ols after veraison
2.4. Changes in the proportions of total monomers, total oligomers, and total polymers after veraison
3. Experimental
3.1. Chemicals and standards
3.2. Preparation of samples
3.3. Extraction and purification of grape seed flavan-3-ols
3.4. Flavan-3-ol analysis by normal phase HPLC-MS
3.5. Statistical analysis
4. Conclusions
Acknowledgements
- Samples Availability: Contact the authors.
References and Notes
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar]
- Jordão, A.M.; Ricardo-da-silva, J.M.; Laureano, O. Evolution of catechins and oligomeric procyanidins during grape maturation of Castelão Francês and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar]
- Vivas, N.; Nonier, M.F.; Vivas De Gaulejac, N.; Absalon, C.; Bertrand, A.; Mirabel, M. Differentiation of proanthocyanidin tannins from seeds, skins and stems of grapes (Vitis vinifera) and heartwood of Quebracho (Schinopsis balansae) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thioacidolysis/liquid chromatography/electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 513, 247–256. [Google Scholar] [CrossRef]
- Singleton, V.L.; Draper, D.E. The transfer of polyphenolic compounds from grape seeds into wine. Am. J. Enol. Vitic. 1964, 15, 34–40. [Google Scholar]
- Sun, B.S.; Pinto, T.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am. J. Enol. Vitic. 1999, 50, 179–184. [Google Scholar]
- Robichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 1990, 53, 343–353. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1999, 4, 74–95. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Agric. Food Chem. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A. The Chemistry of Wine Stabilization and Treatments. In Handbook of Enology, 2nd ed; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; Volume 2, pp. 181–183. [Google Scholar]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A. The Chemistry of Wine Stabilization and Treatments. In Handbook of Enology, 2nd ed; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; Volume 2, pp. 164–166. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefèbvre, S.; Cheynier, V.; Moutounet, M. Influence of fining with different molecular weight gelatins on proanthocyanidin composition and perception of wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of grape and apple proanthocyaindins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 64–573. [Google Scholar] [CrossRef]
- Thorngate, J.H. Methods for analyzing phenolics in research. Am. J. Enol. Vitic. 2006, 57, 269–279. [Google Scholar]
- Yanagida, A.; Shoji, T.; Shibusawa, Y. Separation of proanthocyanidins by degree of polymerization by means of size-exclusion chromatography and related techniques. J. Biochem. Biophys. Methods 2003, 56, 311–322. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Yanagida, A.; Kanda, T.; Shoji, T.; Ohnishi-Kameyama, M.; Nagata, T. Fractionation of apple procyanidins by size-exclusion chromatography. J. Chromatogr. A. 1999, 855, 181–190. [Google Scholar] [CrossRef]
- Sun, B.S.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Separation of grape and wine proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1998, 46, 1390–1396. [Google Scholar] [CrossRef]
- Labarbe, B.; Cheynier, V.; Brossaud, F.; Souquet, J.M.; Moutounet, M. Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1999, 47, 2719–2723. [Google Scholar] [CrossRef]
- Saucier, C.; Mirabel, M.; Daviaud, F.; Longieras, A.; Glories, Y. Rapid fractionation of grape seed proanthocyanidins. J. Agric. Food Chem. 2001, 49, 5732–5735. [Google Scholar] [CrossRef]
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar]
- Gu, L.W.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J.Agric.Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 55–59. [Google Scholar]
- Geny, L.; Saucier, C.; Bracco, S.; Daviaud, F.; Glories, Y. Composition and cellular localization of tannins in grape seeds during maturation. J. Agric. Food Chem. 2003, 51, 8051–8054. [Google Scholar] [CrossRef]
- Romeyer, F.M.; Macheix, J.J.; Sapis, J.C. Changes and importance of oligomeric procyanidins during maturation of grape seeds. Phytochemistry 1986, 25, 219–221. [Google Scholar]
- Fuleki, T.; Ricardo da Silva, J.M. Catechin and procyanidin composition of seeds from grape cultivars grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y.; Monique, A. Developmental changes of procyanidins in grapes of red vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Gaulejac, N.; Augustin, M.; Vivas, N.; Glories, Y. A biochemical approach to the evolution of procyanidins in grape seeds during the ripening of red grapes (Vitis vinifera L. cv. Merlot Noir). J. Wine Res. 1997, 8, 159–167. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000b, 6, 244–254. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.; Shi, Y.; Duan, C. Biosynthesis and Genetic Regulation of Proanthocyanidins in Plants. Molecules 2010, 13, 2674–2703. [Google Scholar]
- Saucier, C.; Bourgeois, G.; Vitry, C.; Roux, D.; Glories, Y. Characterization of (+)-catechin-acetaldehyde polymers: A model for colloidal state of wine polyphenols. J. Agric. Food Chem. 1997, 45, 1045–1049. [Google Scholar]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Competition between (+)-catechin and (-)-epicatechin in acetaldehyde-induced polymerization of flavanols. J. Agric. Food Chem. 1999, 47, 2088–2095. [Google Scholar]
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Santos Buelga, C.; De Freitas, V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003, 68, 476–481. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Pan, Q.; Cui, X.; and Duan, C. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg ×Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.-X.; Pan, Q.-H.; Yan, G.-L.; He, J.-J.; Duan, C.-Q. Changes of Flavan-3-ols with Different Degrees of Polymerization in Seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ Grapes after Veraison. Molecules 2010, 15, 7763-7774. https://doi.org/10.3390/molecules15117763
Liu Y-X, Pan Q-H, Yan G-L, He J-J, Duan C-Q. Changes of Flavan-3-ols with Different Degrees of Polymerization in Seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ Grapes after Veraison. Molecules. 2010; 15(11):7763-7774. https://doi.org/10.3390/molecules15117763
Chicago/Turabian StyleLiu, Yan-Xia, Qiu-Hong Pan, Guo-Liang Yan, Jian-Jun He, and Chang-Qing Duan. 2010. "Changes of Flavan-3-ols with Different Degrees of Polymerization in Seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ Grapes after Veraison" Molecules 15, no. 11: 7763-7774. https://doi.org/10.3390/molecules15117763
APA StyleLiu, Y.-X., Pan, Q.-H., Yan, G.-L., He, J.-J., & Duan, C.-Q. (2010). Changes of Flavan-3-ols with Different Degrees of Polymerization in Seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ Grapes after Veraison. Molecules, 15(11), 7763-7774. https://doi.org/10.3390/molecules15117763





