Synthesis and Antitumor Activity of Amino Acid Ester Derivatives Containing 5-Fluorouracil
Abstract
:1. Introduction
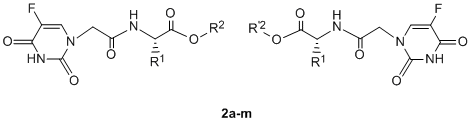
2. Results and Discussion
2.1. Chemistry
2.2. In vitro antitumor activity
3. Experimental
3.1. General
3.2. General procedure for the synthesis of compounds 2a-o
4. Conclusions
Acknowledgements
References
- Smith, N.F.; Figg, W.D.; Sparreboom, A. Recent advances in pharmacogenetic approaches to anticancer drug development. Drug Develop. Res. 2004, 62, 233–253. [Google Scholar] [CrossRef]
- Ragnhammar, P.; Blomgren, H.A. How to optimize the effect of 5-fluorouracil modulated therapy in advanced colorectal cancer. Med. Oncol. 1995, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules 2008, 13, 2340–2369. [Google Scholar] [CrossRef] [PubMed]
- Nichifor, M.; Schacht, E.H. Synthesis of peptide derivatives of 5-fluorouracil. Tetrahedron 1994, 50, 3747–3760. [Google Scholar] [CrossRef]
- Sloan, K.B.; Wasdo, S. Designing for topical delivery: Prodrugs can make the difference. Med. Res. Rev. 2003, 23, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Kumar, B.D.; Karthikeyan, R.S. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef]
- Lee, S.M; Lee, N.J.; Ha, C.K.; Cho, W.J. Syntheses and biological activities of polymers containing methacryloyl-2-oxy-1,2,3-propanetricarboxylic acid of 5-fluorouracil. J. Appl. Polym. Sci. 2004, 94, 57–64. [Google Scholar] [CrossRef]
- Nichifor, M; Schacht, E.H.; Seymour, L.W. Polymeric prodrug of 5-fluorouracil. J. Control. Release 1997, 48, 165–178. [Google Scholar] [CrossRef]
- Yang, Y.W.; Lee, J.S.; Kim, I.; Jung, Y.J.; Kim, Y.M. Synthesis and properties of N-nicotinoyl-2-(5-fluorouracil-1-yl)-D,L-glycine ester as a prodrug of 5-fluorouracil for rectal administration. Er. J. Pharm. Biopharm. 2007, 66, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Elorza, B.; Elorza, M.A.; Frutos, G.; Chantres, J.R. Characterization of 5-fluorouracil loaded liposomes prepared by reverse-phase evaporation or freezing-thawing extrusion methods: Study of drug release. Biochim. Biophys. Acta 1993, 1153, 135–142. [Google Scholar] [CrossRef]
- Kratz, F.; Müller, L.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. ChemMedChem 2008, 3, 20–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Chen, R.Y.; Yang, Y.Y. Synthesis and anticancer activities of novel 5-fluorouracil-1-yl phosphonotripeptides. Chem. J. Chin. Univ. 2002, 23, 1299–1303. [Google Scholar]
- Yin, P.; Hu, M.L.; Hu, L.C. Synthesis, structural characterization and anticarcinogenic activity of a new Gly-Gly dipeptide derivative: Methyl 2-(2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido) acetate. J. Mol. Struct. 2008, 882, 75–79. [Google Scholar] [CrossRef]
- Xiong, J.; Lei, X.X.; Hu, M.L.; Yuan, J.X.; Cai, X.Q. Crystal structure of 2-(5-fluoro-2,4-dioxo-1,2,3,4-tetrahydropyrimidine) glycin, C8H8FN3O5. Z. Kristallogr. NCS. 2006, 221, 37–38. [Google Scholar] [CrossRef]
- Yin, P.; Hu, M.L.; Xiong, J.; Yuan, J.X. Crystal structure of 2(R)-(5-fluorouracil-1-yl)acetyl-2-phenylglycin dimethylformamide solvate, C14H12FN3O5·C3H7NO. Z. Kristallogr. NCS. 2006, 221, 39–40. [Google Scholar]
- Yin, P.; Hu, M.L.; Xiong, J.; Cai, X.Q. Methyl (2S)-2-[2-(5-fluoro-2,4-dioxo-3,4-dihydropy-rimidin-1-yl)acetamido] propionate monohydrate. Acta Crystallogr. E 2006, E62, o1745–o1746. [Google Scholar] [CrossRef]
- Kosynkina, L.; Wang, W.; Liang, T.C. A Convenient Synthesis of Chrial Peptide Nucleic Acid (PNA) Monomers. Tetrahedron Lett. 1994, 35, 5173–5176. [Google Scholar] [CrossRef]
- König, W.; Geiger, R. A new method for synthesis of peptides: activation of the carboxyl group with dicyclohexyl carbodiimide using 1-hydroxybenzotriazoles as additives. Chem. Ber. 1970, 103, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, V.V.; Gopi, H.N. Rapid and efficient synthesis of peptide fragments containing α- aminoisobutyric acid using fmoc-amino acid chlorides/potassium salt of 1-hydroxybenzotriazole. Tetrahedron Lett. 1998, 39, 1049–1050. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, Y.A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron 2004, 60, 2427–2467. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Sashida, Y.; Hirano, T.; Oka, K.; Dobashi, A. Novel cholestane glycosides form the bulbs of ornithogalum saundersiae and their cytostatic activity on leukemia HL-60 and Molt-4 Cells. Tetrahedron 1997, 53, 11549–11562. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Nat. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Hatta, H.; Nishimoto, S.I. Stereoelectronic effect on one-electron reductive release of 5-fluorouracil from 5-fluoro-1-(2’-oxocycloalkyl)uracil as a new class of radiation-actvivated antitumor prodrugs. J.Org. Chem. 2000, 65, 4641–4647. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Compounds | Concentration (mol/L) | ||||
|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |
| 2a | 2.0 | 8.0 | 9,6 | 6.9 | 8.3 |
| 2b | 3.7 | 5.2 | 6.2 | 9.7 | 2.8 |
| 2c | 33.1 | 0.1 | 9.9 | 9.8 | 0 |
| 2d | 27.3 | 0 | 1.7 | 0 | 10.0 |
| 2e-1 | 22.4 | 11.2 | 7.1 | 4.2 | 1.2 |
| 2e-2 | 18.5 | 15.3 | 3.4 | 10.5 | 0 |
| 2f-1 | 3.3 | 10.8 | 6.6 | 12.3 | 0 |
| 2f-2 | 31.8 | 9.2 | 3.7 | 6.2 | 0 |
| 2g | 36.1 | 10.0 | 5.7 | 5.0 | 2.5 |
| 2h | 55.7 | 19.6 | 23.1 | 2.3 | 8.6 |
| 2i | 2.8 | 6.7 | 1.5 | 8.3 | 0.6 |
| 2j | 55.8 | 12.8 | 2.7 | 5.4 | 5.9 |
| 2k-1 | 29.2 | 0 | 3.8 | 8.2 | 2.4 |
| 2k-2 | 51.2 | 15.5 | 9.8 | 12.7 | 8.9 |
| 2l | 42.4 | 7.9 | 5.2 | 9.9 | 5.7 |
| 2m | 65.1 | 0 | 12.6 | 13.0 | 0.3 |
| 2n | 11.4 | 0 | 0 | 0 | 0 |
| 2o | 22.4 | 11.2 | 7.1 | 4.2 | 1.2 |
| 5-FU | 57.4 | 33.5 | 0 | 7.0 | 10.4 |
| FT-207 | 0 | 0 | 0 | 0 | 0 |
| Compounds | Concentration (mol/L) | ||||
|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |
| 2a | 0 | 0 | 0 | 0 | 0 |
| 2b | 9.0 | 8.1 | 0 | 0 | 1.8 |
| 2c | 50.0 | 13.2 | 5.7 | 5.2 | 0 |
| 2d | 13.2 | 0 | 0 | 0 | 0 |
| 2e-1 | 41.2 | 9.7 | 8.8 | 8.2 | 9.1 |
| 2e-2 | 38.4 | 9.1 | 8.8 | 0 | 5.3 |
| 2f-1 | 17.4 | 10.5 | 16.6 | 14.0 | 4.6 |
| 2f-2 | 41.2 | 9.8 | 0 | 0 | 0 |
| 2g | 36.1 | 11.1 | 7.0 | 7.4 | 2.4 |
| 2h | 52.6 | 15.9 | 2.4 | 0.7 | 0 |
| 2i | 14.5 | 8.7 | 8.0 | 4.6 | 11.7 |
| 2j | 34.0 | 9.2 | 4.1 | 3.5 | 5.9 |
| 2k-1 | 35.9 | 0 | 6.9 | 3.0 | 0.7 |
| 2k-2 | 22.4 | 11.9 | 7.1 | 4.2 | 1.2 |
| 2l | 36.2 | 10.2 | 4.9 | 0.5 | 0 |
| 2m | 71.7 | 68.3 | 60.4 | 43.1 | 24.3 |
| 2n | 8.2 | 4.5 | 5.2 | 0 | 0 |
| 2o | 9.7 | 0 | 8.8 | 8.2 | 9.1 |
| 5-FU | 72.6 | 53.8 | 35.0 | 23.8 | 16.6 |
| FT-207 | 58.0 | 8.1 | 0 | 0 | 0 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, J.; Zhu, H.-F.; Zhao, Y.-J.; Lan, Y.-J.; Jiang, J.-W.; Yang, J.-J.; Zhang, S.-F. Synthesis and Antitumor Activity of Amino Acid Ester Derivatives Containing 5-Fluorouracil. Molecules 2009, 14, 3142-3152. https://doi.org/10.3390/molecules14093142
Xiong J, Zhu H-F, Zhao Y-J, Lan Y-J, Jiang J-W, Yang J-J, Zhang S-F. Synthesis and Antitumor Activity of Amino Acid Ester Derivatives Containing 5-Fluorouracil. Molecules. 2009; 14(9):3142-3152. https://doi.org/10.3390/molecules14093142
Chicago/Turabian StyleXiong, Jing, Hai-Feng Zhu, Ya-Juan Zhao, Yun-Jun Lan, Ji-Wang Jiang, Jing-Jing Yang, and Shu-Feng Zhang. 2009. "Synthesis and Antitumor Activity of Amino Acid Ester Derivatives Containing 5-Fluorouracil" Molecules 14, no. 9: 3142-3152. https://doi.org/10.3390/molecules14093142