Abstract
(7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid methyl ester (1) upon reaction with ethyl bromoacetate furnishes (7-ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (2), which on treatment with 100% hydrazine hydrate yields (7-hydrazinocarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide (3). The condensation of compound 3 with different aromatic aldehydes afforded a series of [7-(arylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid arylidene-hydrazide Schiff’s bases 4a-k. Cyclo-condensation of compounds 4a-k with 2-mercapto-acetic acid in N,N-dimethylformamide in the presence of anhydrous ZnCl2 affords N-(2-aryl-4-oxothiazolidin-3-yl)-2-(4-(2-aryl-4-oxothiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a-k. Structure elucidation of the products has been accomplished on the basis of elemental analysis, IR, 1H-NMR and 13C-NMR data. Compounds 4a-k and 5a-k will be screened for their antibacterial activity against both Gram-positive and Gram-negative bacteria and the results reported elsewhere in due course.
Introduction
Coumarin (2-oxo-2H-chromene) and its derivatives represent one of the most active classes of heterocyclic compounds, possessing a wide spectrum of biological activities [,,,,,,,,] as many of these compounds posses antitumor [,], antibacterial [,], antifungal [,,], anticoagulant [] and anti-inflammatory [] properties. They have also shown to be useful as anti-HIV agents and as CNS active compounds []. In addition, these compounds are used as additives to food and cosmetics [], dispersed fluorescence and lasers []. Coumarins are present in remarkable amounts in plants, although their presence has also been detected in microorganisms and animal sources [].
Thiazolidinones are derivatives of thiazolidine and they also constitute an important group of heterocyclic compounds. Thiazolidinones, with a carbonyl group in positions 2,4- or just 4-, have been subject of extensive study in the recent past [,] and literature surveys show that thiazolidin-4-ones are important compounds due to their broad range of biological activities [,,,,,,]. 4-Thiazolidinones substituted in the 2-position, its derivatives and analogues exhibit unusually high in vitro activity against Mycobacterium tuberculosis []. Overviews of their synthesis, properties, reactions and applications have been published [,].
Thiazolidine compounds are formed by condensation of either aliphatic or aromatic moieties, containing a formyl group (-CHO), with different aminothiols []. It should be noted that the thiazolidine ring is the core building unit of penicillin antibiotics. A novel synthesis of thiazolidine-2-thione and thiazolidine-2-one derivatives is described with the iodo-cyclothiocarbamation reaction as the key step for the heterocyclic ring formation. The new method has been applied to the synthesis of thiazolidinones as bioisosteric analog of Linezolid []. In recent years several new methods for preparing thiazolidinone derivatives have been reported in the literature [,].
As part of our aim in search of biologically active heterocyclic compounds with one, two or three coumarin cores and thiazolidinone moieties, we have previously reported the synthesis of some of these compounds [,]. Since 4-thiazolidinones show a wide range of biological activities [,,,,,,], we extended our work on the synthesis of novel compounds formed by cyclocondensation from compounds (Schiff’s bases) 4a-k and mercaptoacetic acid in N,N-dimethylforamide in the presence of anhydrous ZnCl2, to afford N-(2-aryl-4-oxothiazolidin-3-yl)-2-(4-(2-aryl-4-oxothiazolidin-3-yl-carbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a-k (Scheme 1).
Thiazolidinone coumarin derivatives have been proven to have significant biological activity, like anticonvulsant activity [], cytotoxic activity [] and antioxidant activity []. Therefore, our aim in this work was to prepare thiazolidinone derivatives using (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid as starting compound.
Results and Discussion
Synthesis
The synthesis of the target compounds was carried out as outlined in Scheme 1. The starting compound (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid methyl ester (1) was prepared in 92% yield by esterification of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid []. We have previously reported the preparation of (7-ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methyl ester (2) in 82% yield by direct condensation of 1 with bromoacetic acid ethyl ester [].
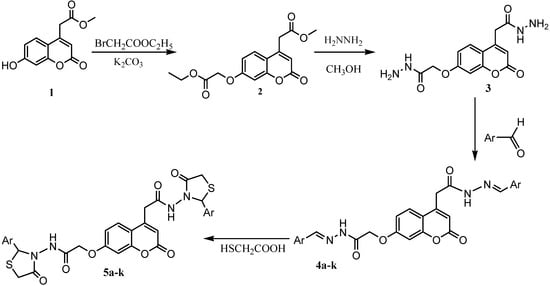
Scheme 1.
Synthetic route for thiazolidinones 5a-k.
Hydrazinolysis of 2 with 86% hydrazine hydrate in methanol at room temperature afforded dihydrazide 3 in good yield. The FT-IR spectra of carbohydrazide 3 showed absorption bands in the 3,317 cm-1 (hydrazide NH-NH2), 3,269 cm-1(aromatic C-H), 1,711 cm-1(-C=O carbonyl stretching) and 1,621-1,640 cm-1(-CO-NH-NH2 groups) regions, respectively. The 1H-NMR spectrum exhibited a singlet due to the –CO-NH-NH2 NH proton at δ 9.32 ppm. Methylene protons –CH2 and –OCH2 resonated as singlets at 4.23 and 4.85 ppm, respectively.
A new series of compounds 4a-k was prepared similarly to those previously described [] by refluxing a solution of suitable different aromatic aldehydes and dihydrazide 3 in absolute ethanol for 2 to 4 hours, in a presence of a catalytic amount of glacial acetic acid. The structures of compounds 4a-k were inferred from their analytical and spectral data.
Thus, their IR spectra showed characteristic bands at 3,448-3,278 cm-1(NH), 1,709 cm-1, 1,672 cm-1 (C=O, lactone) and 1,620 cm-1(C=O, amide, -HC=N- azomethine). The 1H-NMR spectra did not only show the absence of NH2 protons at 3.38, but also the presence of the N=CH proton at 8.30 ppm.
N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-(2-aryl-4-oxo-thiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a-k were obtained by refluxing a solution of compounds 4a-k and thioglicolic acid in N,N-dimethylformamide for 6-8 hours in the presence of anhydrous ZnCl2.
Experimental
General
Melting points were determined on Electrothermal Capillary melting point apparatus and are uncorrected. Thin-layer chromatography was performed with fluorescent silica gel plates HF254 Merck, which were checked under UV 254 and 365 nm light. The elemental analysis for C, H and N was done on a Perkin-Elmer Analyzer 2440. Infrared spectra (νmax-cm-1) were recorded on a Beckmann FT-IR 3303, using KBr disks. 1H-NMR spectra were recorded on JEOL EX-270 MHz NMR Spectrometer at 293 K in DMSO-d6. 13C-NMR spectra were recorded on a Varian Gemini at 50 MHz in DMSO-d6. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS.
(7-Ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (2)
A mixture of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (1, 25.74 g, 0.11 mole), anhydrous potassium carbonate (15.20 g, 0.11 mole) and ethyl bromoacetate (18.37 g, 0.11 mole) in dry acetone (200 mL) was refluxed with continuous stirring for 12 hours. After filtration, the solution was concentrated under reduced pressure, vacuum dried and the solid product was recrystallized from ethanol.M.p.185-186◦C, yield 64%; IR: νmax 3,429, 2,986, 2,941, 1,753, 1,724, 1,619, 1,439, 1,393, 1,341, 1,221, 1,198, 1,089 cm-1; 1H-NMR: δ 7.76 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.92 (s, 2H, -OCH2), 4.19 (q, 2H, CH2, -CH2CH3), 4.02 (s, 2H, CH2), 3.65 (s, 3H, OCH3), 1.22 (t, 3H, CH3, -CH2CH3); 13C-NMR: δ 14.2 (CH2CH3), 34.8 (CH2CO), 52.1 (OCH3), 61.3 (CH2CH3), 65.5 (COCH2O), 109.6 (C-8), 112.8 (C-6), 113.8 (C-3), 114.8 (C-10), 128.3 (C-5), 151.2 (C-9), 155.2 (C-4), 160.3 (C-7), 160.9 (C-2), 168.9 (CO-O), 169.3 (C-CO-C); Anal. Calcd. for C16H16O7: C, 60.00; H, 5.04; Found: C, 59.98; H, 5.01%.
(7-Hydrazinocarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide (3)
To a solution of methanol (120 mL) and 86% hydrazine hydrate (12 mL) (7-ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (2, 3.2 g, 0.01 mole) was added, and the mixture was left to stand overnight at 5◦C. The product precipitated and was collected by suction filtration, washed with methanol (petrolether) and recrystallized from dil. acetic acid. M.p.> 300◦C, yield 70%; IR: νmax 3,461, 3,325 (NH), (NH2), 1,707 (lactone C=O), 1,623 (C=O, amide), 1,516 (C=C, arom.), 1,430, 1,298, 1,277 and 1,153 cm-1; 1H-NMR: δ 9.41 (s, 1H, NH), 9.34 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.94 (s, 2H, -OCH2), 4.34 (s, 2H, NH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, NH2); 13C-NMR: δ 45.8 (CH2), 68.9 (CH2O-), 108.0 (C-8), 111.8 (C-6), 112.9 (C-3), 114.1 (C-10), 128.3 (C-5), 152.2 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 169.6 (COCH2); Anal. Calcd. for C13H14N4O5: C, 50.98; H, 4.61; N, 18.29; Found: C, 51.02; H, 4.58; N, 18.25%.
General procedure for preparation of (7-(arylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl)-acetic acid aryilidene-hydrazides 4a-k
A mixture of (7-Hydrazinocarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide (3, 3.06 g, 0.01 mole) and appropriate aromatic aldehyde (Ar/a-k, 0.01 mole) was refluxed in absolute ethanol (30 mL) in the presence of a catalytic amount of glacial acetic for 2 to 4 hours. The reaction mixture was cooled, the solid separated was filtered and recrystallized from methanol to give compounds 4a-k.
[7-(Benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid benzylidene hydrazide (4a). M.p. 268-269◦C; yield 74%; IR: νmax 3,418, 3,313 (NH), 1,712, 1,682 (C=O, lactone), 1,666 (C=O, amide), 1,613 (C=C, arom., C=N, azomet.), 1,550, 1,378, 1,269 and 1,153 cm-1; 1H- NMR: δ 8.30 (s, 1H, HC=N-), 8.24 (s, 1H, HC=N-), 8.06 (s, 1H, NH), 8.02 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.72-7.31 (m, 10H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.83 (s, 2H, -OCH2), 4.28 (s,2H,CH2); 13C-NMR: δ 45.7 (CH2), 69.2 (CH2O-), 108.1 (C-8), 111.6 (C-6), 112.5 (C-3), 114.4 (C-10), 127.9 (C-5), 128.4 (C-3,5, Ar-), 129.0 (C-2,6, Ar-), 131.4 (C-4, Ar-), 133.9 (C-1, Ar-), 143.6 (N=CH-), 151.8 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.9 (COCH2O), 170.0 (CONH-); Anal. Calcd. for C27H22N4O5: C, 67.21; H, 4.60; N, 11.61; Found: C, 67.19; H, 4.61; N, 11.58%.
[7-(2-Chlorobenzylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2-chloro-benzylidene)- hydrazide (4b). M.p. 225-226◦C, yield (76%); IR: νmax 3,428, 3,283 (NH), 1,710, 1,692 (C=O, lactone), 1,656 (C=O, amide), 1,612 (C=C, arom., C=N, azomet.), 1,542, 1,398, 1,264 and 1,155 cm-1; 1H-NMR: δ 8.73 (s, 1H, -HC=N-), δ 8.62 (s, 1H, -HC=N-), δ 8.48 (s, 1H, NH), 8.45 (s, 1H, NH), 7.72 (d, 1H, H-5), 7.60-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.30 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 68.9 (CH2O-), 107.8 (C-8), 111.5 (C-6), 112.7 (C-3), 113.9 (C-10), 127.8 (C-5), 129.4 (C-3, Ar-), 130.6 (C-6, Ar-), 132.7 (C-4, Ar-), 133.8 (C-1, Ar-), 134.3 (C-2, Ar-), 143.5 (N=CH-), 151.7 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 169.9 (CONH-); Anal. Calcd. For C27H20Cl2N4O5: C, 58.81; H, 3.66; N, 10.16; Found: C, 58.79; H, 3.69; N, 10.12%.
[7-(3-Chlorobenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (3-chloro-benzylidene)-hydrazide (4c). M.p. 259-261◦C, yield 72%; IR: νmax 3,408, 3,188 (NH), 1,727, 1,683 (C=O, lactone), 1,616 (C=O, amide, C=N, azomet.), 1,561 (C=C, arom.), 1,394, 1,262 and 1,138 cm-1; 1H-NMR: δ 8.31 (s, 1H, -HC=N-), 8.22 (s, 1H, -HC=N-), 8.48 (s, 1H, NH), 8.45 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.67-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.28 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 69.1 (CH2O-), 107.9 (C-8), 111.4 (C-6), 112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.9 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C-4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.4 (N=CH-), 151.6 (C-9), 155.2 (C-4), 160.6 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C27H20Cl2N4O5: C, 58.81; H, 3.66; N, 10.16; Found: C, 58.80; H, 3.68; N, 10.13%.
[7-(2,4-Dihydroxy-benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2,4-dihydroxybenzylidene)-hydrazide (4d). M.p. 273-275◦C, yield 52%; IR: νmax 3,434 (OH), 3,366, 3,092 (NH), 1,712, 1,672 (C=O, lactone), 1,623 (C=O, amide, C=N, azomethine), 1,612 (C=C, arom., C=N), 1,559, 1,509, 1,395, 1,265 and 1,153 cm-1; 1H-NMR: δ 11.80 (s, 1H, OH), 11.17 (s, 1H, OH), 8.42 (s, 1H, -HC=N-), 8.30 (s, 1H, -HC=N-), 8.23 (s, 1H, NH), 8.19 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.61-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.82 (s, 2H, -OCH2), 4.28 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.2 (CH2O-), 103.8 (C-3, Ar-), 107.6 (C-8), 108.7 (C-5, Ar-), 111.3 (C-6), 112.7 (C-3), 113.4 (C-10), 127.2 (C-6, Ar-), 127.9 (C-5), 135.2 (C-1, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.1 (C-4), 160.5 (C-7), 160.9 (C-2), 162.4 (C-2, Ar-), 162.6 (C-4, Ar-), 166.5 (COCH2O), 169.4 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.30; H, 4.07; N, 10.29%.
[7-(3,4-Dihydroxy-benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]- acetic acid (3,4-dihydroxybenzylidene)-hydrazide (4e). M.p. 205◦C, yield 62%; IR: νmax 3,408, 2,922 (NH), 1,725, 1,664 (C=O, lactone), 1,619 (C=O, amide, C=N, azomethine), 1,593 (C=C, arom.), 1,444, 1,393, 1,284 and 1,152 cm-1; 1H-NMR: δ 11.98 (s, 1H, OH), 11.45 (s, 1H, OH), 8.41 (s, 1H, -HC=N-), 8.30 (s, 1H, -HC=N-), 8.12 (s, 1H, NH), 8.03 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.65-7.41 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.77 (s, 2H, -OCH2), 4.22 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 69.3 (CH2O-), 107.6 (C-8), 111.4 (C-6), 112.5 (C-3), 113.5 (C-10), 116.4 (C-2, Ar-), 117.5 (C-5, Ar-), 123.3 (C-6, Ar-), 127.8 (C-5), 127.9 (C-1, Ar-), 143.1 (N=CH-), 147.4 (C-3, Ar-), 149.6 (C-4, Ar-), 151.3 (C-9), 155.0 (C-4), 160.4 (C-7), 160.9 (C-2), 166.6 (COCH2O), 169.5 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.13; H, 4.03; N, 10.04%.
[7-(2,5-Dihydroxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]- acetic acid (2,5-dihydroxybenzylidene)-hydrazide (4f). M.p. 275-276◦C, yield 76%; IR: νmax 3,369, 3,286 (NH), 1,717, 1,681, 1,667 (C=O, lactone), 1,624 (C=O, amide, C=N, azomethine), 1,585 (C=C arom.), 1,492, 1,396, 1,267 and 1,156 cm-1; 1H-NMR: δ 11.95 (s, 1H,OH), 11.56 (s,1H,OH), 8.48 (s, 1H, -HC=N-), 8.34 (s, 1H, -HC=N-), 8.30 (s, 1H, NH), 8.25 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.68-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.82 (s, 2H, -OCH2), 4.24 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.3 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.7 (C-3), 113.8 (C-10), 116.4 (C-6, Ar-), 117.4 (C-3, Ar-), 119.6 (C-4, Ar-), 119.9 (C-1, Ar-), 127.8 (C-5), 143.5 (N=CH-), 151.4 (C-9), 151.3 (C-5, Ar-), 153.7 (C-2, Ar-), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.4 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.32; H, 4.04; N, 10.20%.
[7-(4-Hydroxy-3-methoxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4yl]-acetic acid(4-hydroxy-3-methoxybenzylidene)-hydrazide (4g). M.p. 232-233◦C, yield 84%; IR: νmax 3,430, 3,224 (NH), 1,711, 1,671 (C=O, lactone), 1,622 (C=O, amide, C=N, azomethine), 1,605 (C=C, arom.), 1,429, 1,394, 1,272 and 1,164 cm-1; 1H-NMR: δ 11.96 (s, 1H, OH), δ 11.50 (s, 1H, OH), 8.19 (s, 1H, -HC=N-), 8.10 (s, 1H, -HC=N-), 7.99 (s, 1H, NH), 7.97 (s, 1H, NH), 7.77 (d, 1H, H-5), 7.40-7.21 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.78 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.80 (s, 6H, -OCH3); 13C-NMR: δ 45.6 (CH2), 56.0 (OCH3), 69.2 (CH2O-), 107.6 (C-8), 111.2 (C-6), 112.5 (C-3), 113.6( C-10), 114.8 (C-2, Ar-), 117.0 (C-5, Ar-), 122.9 (C-6, Ar-), 127.4 (C-1,Ar-), 127.8 (C-5), 143.3 (N=CH-), 148.1 (C-4, Ar-), 151.4 (C-9), 151.5 (C-3, Ar-), 155.1 (C-4), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C29H26N4O9: C, 60.62; H, 4.56; N, 9.75; Found: C, 60.59; H, 4.75; N, 9.70%.
[7-(3-Phenoxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid 3-phenoxybenzylidene)-hydrazide (4h). M.p. 236-237◦C, yield 57%; IR: νmax 3,409, 3,071 (NH), 1,726, 1,685 (C=O, lactone), 1,624 (C=O, lactone, C=N, azomethine), 1,597 (C=C, arom.), 1,490, 1,394, 1,261 and 1,156 cm-1; 1H-NMR: δ 8.30 (s, 1H, -HC=N-), 8.21 (s, 1H, -HC=N-), 8.03 (s, 1H, NH), 7.99 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.70-7.10 (m, 18H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.79 (s, 2H, -OCH2), 4.18 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 56.1 (OCH3), 69.1 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.7 (C-3), 113.5 (C-10), 116.6 (C-2, Ar-), 117.5 (C-2,6, Ar-PhO), 119.8 (C-4, Ar-), 121.9 (C-4, Ar- PhO), 122.3 (C-2, Ar-), 127.8 (C-5), 128.5 (C-3,5 Ar- PhO), 128.9 (C-5 Ar-), 133.5 (C-1 Ar-), 143.4 (N=CH-), 151.3 (C-9), 155.4 (C-4), 157.1 (C-1 Ar- PhO), 157.1 (C-3 Ar-), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 170.0 (CONH-); Anal. Calcd. For C39H30N4O7: C, 70.26; H, 4.54; N, 8.40; Found: C, 70.23; H, 4.55; N, 8.37%.
[7-(4-N,N-Dimethylaminobenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (4-N,N-dimethylaminobenzylidene)-hydrazide (4i). M.p. 207-209◦C, yield 63%; IR: νmax 3,408, 3,082 (NH), 1,724, 1,679 (C=O, lactone), 1,623 (C=O, amide, C=N, azomethine), 1,604 (C=C, arom.), 1,554, 1,525, 1,364, 1,269 and 1,181cm-1; 1H-NMR: δ 8.49 (s, 1H, -HC=N-), 8.44 (s, 1H, -HC=N-), 8.17 (s, 1H, NH), 8.07 (s, 1H, NH), 7.66 (d, 1H, H-5), 7.24-7.52 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.74 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.32 (s, 6H, -N(CH3)2), 2.99 (s, 6H, -N(CH3)2); 13C-NMR: δ 40.3 (CH3N-), 45.6 (CH2), 69.1 (CH2O-), 107.6 (C-8), 111.3 (C-6), 112.7 (C-3), 113.8 (C-10), 114.4 (C-3,5 Ar-), 123.3 (C-1, Ar-), 127.8 (C-5), 130.2 (C-2,6 Ar-), 143.3 (N=CH-), 151.4 (C-9), 151.0 (C-4, Ar-), 155.5 (C-4), 160.6 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C31H32N6O5: C, 64.97; H, 5.45; N, 15.15; Found: C, 65.89; H, 5.58; N, 15.11 %.
[7-(2-Hydroxy-5-nitrobenzylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2-hydroxy-5-nitrobenzylidene)-hydrazide (4j). M.p. 204◦C, yield 82%; IR: νmax 3,367, 3,272 (NH), 1,706, 1,689 (C=O, lactone), 1,616 (C=O, C=N, azomethine), 1,600 (C=C, arom.), 1,577, 1,517, 1,481, 1,342, 1,287 and 1,150 cm-1; 1H-NMR: δ 12.02 (s, 2H, OH), 8.71 (s, 1H, -HC=N-), 8.59 (s, 1H, -HC=N-), 8.36 (s, 1H, NH), 8.31 (s, 1H, NH), 7.67 (d, 1H, H-5), 7.32-7.54 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.08 (s, 2H, CH2); 13C-NMR: δ 45.7 (CH2), 69.2 (CH2O-), 107.8 (C-8), 111.5 (C-6), 112.4 (C-3), 113.8 (C-10), 116.9 (C-3, Ar-), 119.4 (C-1, Ar-), 124.8 (C-4, Ar-), 125.5 (C-2, Ar-), 127.8 (C-5), 141.6 (C-5, Ar-), 143.4 (N=CH-), 151.4 (C-9), 155.4 (C-4), 160.8 (C-7), 160.9 (C-2), 166.2 (C-2, Ar-), 166.8 (COCH2O), 170.0 (CONH-); Anal. Calcd. For C27H20N6O11: C, 53.65; H, 3.33; N, 13.90; Found: C, 53.63; H, 3.35; N, 13.91%.
[2-Oxo-7-(3-phenylallylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (3-phenylallylidene)-hydrazide (4k). M.p. 290-292◦C, yield 68%; IR: νmax 3,428, 3,256 (NH), 1,718 (C=O, lactone), 1,624 (C=O, amide, C=N, azomethine), 1,613 (C=C, arom.), 1,560, 1,509, 1,393, 1,266 and 1,151 cm-1; 1H-NMR: δ 8.38 (s, 1H, -HC=N-), 8.24 (s, 1H, -HC=N-), 8.15 (s, 1H, NH), 8.08 (s, 1H, NH), 7.78 (2d, 4H, -HC=CH-), 7.64 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.77 (s, 2H, -OCH2), 4.08 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.1 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.8 (C-3), 113.4 (C-10), 126.3 (C-2, Ar-), 126.4 (C-2,6, Ar-), 127.8 (C-5), 128.0 (C-4, Ar-), 128.9 (C-3,5, Ar-), 135.1 (C-1, Ar-), 139.0 (C-3, Ar-), 143.3 (N=CH-), 151.2 (C-9), 155.4 (C-4), 160.5 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8,(CONH-); Anal. Calcd. For C31H26N4O5: C, 69.65; H, 4.90; N, 10.48; Found: C, 69.67; H, 4.88; N, 10.45%.
General procedure for the preparation of N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-(2-aryl-4-oxo-thiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a-k
A mixture of (7-(arylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl)-acetic acid aryilidenehydrazide 4a-k (0.01 mole) and mercaptoacetic acid (1.82 g, 0.02 mole) in DMF (30 mL) containing a pinch of anhydrous ZnCl2 was refluxed 6-8 hours. The reaction mixture was cooled and poured onto crushed ice. The solid thus obtained was filtered, washed with water and recrystallized from DMF yielding 5a-k.
2-{2-Oxo-7-[(4-oxo-2-phenylthiazolidin-3-ylcarbamoyl)-methoxy]-2H-chromen-4-yl}-N-(4-oxo-2-phenylthiazolidin-3-yl)acetamide (5a). M.p. 202-204◦C, yield 40%; IR: νmax 3,418, 3,313 (NH), 1,712, 1,682 (C=O, lactone), 1,666 (C=O, amide), 1,613 (C=C, arom.), 1,550, 1,378, 1,269 and 1,153 cm-1; 1H-NMR: δ 8.22 (s, 1H, -NH), 8.12 (s, 1H, -NH), 7.76 (d,1H,H-5), 7.71-7.23 (m, 10H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, -SCHN-), 4.83 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S-); 13C-NMR: δ 35.8 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.2 (C-4, Ar-), 127.8 (C-5), 128.7 (C-3,5, Ar-), 128.8 (C-2,6 Ar-), 139.2 (C-1, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 168.8 (SCH2CO-N), 173.3 (CONH-); Anal. Calcd. For C31H26N4O7S2: C, 59.94; H, 4.16; N, 8.88; S, 10.17; Found: C, 60.05; H, 4.14; N, 8.91; S, 10.14%.
N-[2-(2-Chlorophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(2-chlorophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5b). M.p. 184◦C, yield 76%; IR: νmax 3,425, 3,283 (NH), 1,692 (C=O, lactone), 1,656 (C=O, amide), 1,612 (C=C, arom.), 1,542, 1,398, 1,264 and 1,155 cm-1; 1H-NMR: δ 8.73 (s, 1H, NH-), 8.62 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.60-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.30 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.0 (C-5), 129.0 (C-3, Ar-), 130.6 (C-6, Ar-), 132.5 (C-4, Ar-), 133.4 (C-1, Ar-), 134.0 (C-2, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 173.0 (CONH-); Anal. Calcd. For C31H24Cl2N4O7S2: C, 53.22; H, 3.46; N, 8.01; S, 9.17; Found: C, 53.18; H, 3.44; N, 7.89; S, 9.20%.
N-[2-(3-Chlorophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(3-chlorophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5c). M.p. 240-241◦C, yield 72%; IR: νmax 3,450, 3,188 (NH), 1,727, 1,683 (CO, lactone), 1,616 (C=O, amide), 1,598 (C=C, arom.), 1,394, 1,262 and 1,138 cm-1; 1H-NMR: δ 8.31 (s, 1H, NH-), 8.22,(s, 1H, NH-), 7.76 (d, 1H, H-5), 7.67-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.8 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C-4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 173.0 (COCH2O), 173.0 (CONH-); Anal. Calcd. For C31H24Cl2N4O7S2: C, 53.22; H, 3.46; N, 8.01; S, 9.17; Found: C, 53.18; H, 3.44; N, 7.89; S, 9.20%.
N-[2-(2,4-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(4-{[2-(2,4-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methyl}-2-oxo-2H-chromen-7-yloxy)-acetamide (5d). M.p. 239-241◦C, yield 52%; IR: νmax 3,266 (OH), 3,092 (NH), 1,712, 1,672 (C=O, lactone), 1,624 (C=O, amide), 1,612 (C=C, arom.), 1,559, 1,509, 1,395, 1,265 and 1,153 cm-1; 1H-NMR: δ 11.80 (s, 1H, OH), 11.17 (s, 1H, OH), 8.42 (s, 1H, NH-), 8.30 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.61-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.82 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.4 (NCHS), 69.10 (CH2O-), 103.7 (C-3, Ar-), 107.6 (C-8), 108.4 (C-5, Ar-), 110.1 (C-1, Ar-), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.8 (C-5), 131.3 (C-6, Ar-), 151.2 (C-9), 157.2 (C-2, Ar-), 158.2 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH), 168.8 (NCOCH2), 173.3 (CH2CONH); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(3,4-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(3,4-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5e). M.p. 198-200◦C, yield 47%; IR: νmax 3,388 (NH), 2,922 (OH), 1,725, 1,694 (C=O, lactone), 1,619 (C=O, amide), 1,523, 1,444, 1,393, 1,284 and 1,152 cm-1; 1H-NMR: δ 11.98 (s, 1H, OH), 11.45 (s, 1H, OH), 8.41 (s, 1H, NH), 8.30 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.65-7.41 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.77 (s, 2H, -OCH2), 4.22 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 103.7 (C-3, from Ph), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-2, Ar-), 117.4 (C-5, Ar-), 122.2 (C-6, Ar-), 117.8 (C-5), 133.8 (C-1, Ar-), 143.0 (N=CH-), 147.4 (C-3, Ar-), 145.6 (C-4, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(2,5-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(2,5-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5f). M.p. 221-223◦C, yield 46%; IR: νmax 3,369 (OH), 3,286 (NH), 1,717, 1,681 (C=O, lactone), 1,667 (C=O, amide), 1,624 (C=C, arom.), 1,585, 1,492, 1,396, 1,267 and 1,156 cm-1; 1H-NMR: δ 11.95 (s, 1H, OH), 11.56 (s, 1H, OH), 8.48 (s, 1H, NH-), 8.34 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.68-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.82 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.4 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-6, Ar-), 117.4 (C-3, Ar-), 115.6 (C-4, Ar-), 119.6 (C-1, Ar-), 127.8 (C-5), 143.0 (N=CH-), 148.7 (C-2, Ar-), 151.2 (C-9), 151.2 (C-5, Ar-), 155.0 (C-4), 160.2 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.3 (CONH-); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(4-Hydroxy-3-methoxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(4-hydroxy-3-methoxyphenyl-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5g). M.p. 217-218◦C, yield 84%; IR: νmax 3,434, 3,224 (NH), 1,711, 1,671 (C=O, lactone), 1,632 (C=O, amide), 1,603 (C=C, arom.), 1,529, 1,394, 1,272 and 1,164 cm-1; 1H-NMR: δ 11.96 (s, 1H, OH), 8.19 (s, 1H, NH-), 8.10 (s, 1H, NH-), 7.77 (d, 1H, H-5), 7.40-7.21 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.78 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.80 (s, 6H, -OCH3), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 56.2 (OCH3), 57.8 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 114.8 (C-2, Ar-), 117.0 (C-5, Ar-), 122.9 (C-6, Ar-), 132.4 (C-1, Ar-), 144.1 (C-4, Ar-), 151.2 (C-9), 151.5 (C-3, Ar-), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.0 (CONH-), Anal. Calcd. For C33H30N4O11S2: C, 54.84; H, 4.18; N, 7.75; S, 8.87; Found: C, 54.79; H, 4.19; N, 7.71; S, 8.82%.
2-(2-Oxo-7-{[4-oxo-2-(3-phenoxyphenyl)-thiazolidin-3-ylcarbamoyl]-methoxy}-2H-chromen-4-yl)-N-[4-oxo-2-(3-phenoxyphenyl)-thiazolidin-3-yl]-acetamide (5h). M.p. 221-222◦C, yield 57%; IR: νmax 3,389, 3,071 (NH), 1,726, 1,685 (C=O, lactone), 1,628 (C=O, amide), 1,614 (C=C, arom.), 1,577, 1,490, 1,394, 1,261 and 1,156 cm-1; 1H-NMR: δ 8.30 (s, 1H, NH-), 8.21 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.70-7.10 (m, 18H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.79 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 56.2 (OCH3), 57.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-4, Ar-), 116.1 (C-2, Ar-), 117.5 (C -2,6, Ar- PhO), 121.4 (C-4, Ar- PhO), 121.9 (C-6, Ar-), 127.8 (C-5), 128.5 (C-3,5 Ar- PhO), 128.6 (C-5 Ar-), 139.1 (C-1 Ar-), 151.2 (C-9), 155.0 (C-4), 156.8 (C-3, Ar-), 157.6 (C-1, Ar-PhO), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C43H34N4O9S2: C, 63.38; H, 4.21; N, 6.88; S, 7.87; Found: C, 63.34; H, 4.19; N, 6.86; S, 7.84%.
N-[2-(4-N,N-Dimethylaminophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(4-N,N-dimethylaminophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5i). M.p. 198-201◦C, yield 71%; IR: νmax 3,398, 3,082 (NH), 1,724, 1,679 (C=O, lactone), 1,623 (C=O, amide), 1,604 (C=C, arom.), 1,554, 1,525, 1,364, 1,269 and 1,181 cm-1; 1H-NMR: δ 8.49 (s, 1H, NH-), 8.44 (s, 1H, NH-), 7.66 (d, 1H, H-5), 7.24-7.52 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s,1H,H-3), 5.92 (s, 1H, NCHS), 4.74 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.38 (s, 2H, COCH2S), 3.32 (s, 6H, -N(CH3)2), 2.99 (s, 6H, -N(CH3)2); 13C-NMR: δ 35.7 (COCH2S), δ 40.3 (CH3N-), 45.5 (CH2), 57.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 114.4 (C-3,5, Ar-), 127.8 (C-5), 128.9 (C-1, Ar-), 130.1 (C-2,6 Ar-), 148.2 (C-4, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C35H36N6O7S2: C, 58.64; H, 5.06; N, 11.72; S, 8.95; Found: C, 58.60; H, 4.98; N, 11.70; S, 8.90%.
N-[2-(2-Hydroxy-5-nitrophenyl)-4-oxo-thiazolidin-3-yl]-2-(4-{[2-(2-hydroxy-5-nitrophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methyl}-2-oxo-2H-chromen-7-yloxy)-acetamide (5j). M.p. 240-242◦C, yield 82%; IR: νmax 3,367, 3,272 (NH), 1,689 (C=O), 1,618 (C=O, amide), 1,598 (C=C, arom.), 1,577, 1,517, 1,481, 1,342, 1,287 and 1,150 cm-1; 1H-NMR: δ 12.02 (s, 2H, OH), 8.71 (s, 1H, NH-), 8.59 (s, 1H, NH-), 7.67 (d, 1H, H-5), 7.32-7.54 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C- NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 116.9 (C-3, Ar-), 119.4 (C-1, Ar-), 121.8 (C-4, Ar-), 125.5 (C-6, Ar-), 127.8 (C-5), 141.1 (C-5, Ar-), 151.2 (C-9), 155.0 (C-4) 160.3 (C-7), 160.9 (C-2), 163.2 (C-2 Ar-), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C31H24N6O13S2: C, 49.47; H, 3.21; N, 11.17; S, 8.52; Found: C, 49.45; H, 3.19; N, 11.12; S, 8.50%.
2-{2-Oxo-7-[(4-oxo-2-styrylthiazolidin-3-ylcarbamoyl)-methoxy]-2H-chromen-4-yl}-N-(4-oxo-2-styrylthiazolidin-3-yl)-acetamide (5k). M.p. 221-224◦C, yield 48%; IR: νmax 3,424, 3,276 (NH), 1,718 (C=O, lactone), 1,628 (C=O, amide), 1,613 (C=C, arom.), 1,560, 1,509, 1,393, 1,266 and 1,151 cm-1; 1H-NMR: δ 8.38 (s, 1H, NH-), 8.24 (s, 1H, NH-), 7.78 (2d, 4H, -HC=CH-), 7.64 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.77 (s, 2H, -OCH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 36.3 (COCH2S), δ 45.5 (CH2), 56.9 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10) 123.8 (C-1, ethenyl-Ar)), 126.4 (C-2,6, Ar-), 128.0 (C-4, Ar-), 128.7 (C-3,5, Ar-), 129.6 (C-2, ethenyl-Ar), 135.2 (C-1, Ar-), 137.3 (N=CH-), 139.0 (C-3 Ar-), 151.2 (C-9), 155.0 (C-4), 160.2 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C35 H30 N4 O7S2: C, 61.57; H, 4.43; N, 8.21; S, 9.39;Found: C, 61.56; H, 4.41; N, 8.19; S, 9.40%.
References
- Raev, L.; Voinov, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1990, 45, 696–698. [Google Scholar]
- Nofal, Z.M.; El-Zahar, M.; Abd El-Karim, S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Abd El-Latif, M.S.; El-Hady, N.A.; Fakery, A.H.; Badair, A.H. Heteroaromatisation with 4-hydroxycoumarin part 2. Molecules 2001, 6, 519–527. [Google Scholar] [CrossRef]
- Patibha, S.; Shreeya, P. Synthesis, Characterization and antimicrobial studies of some novel 3-arylazo-7-hydroxy-4-methylcoumarin. Indian J. Chem. 1999, 38B, 1139–1142. [Google Scholar]
- Patonay, T.; Litkei, G.Y.; Bognar, R.; Erdei, J.; Misztic, C. Synthesis, antimicrobial and antifungal activity of 4-hydroxycoumarin derivatives, Analogues of Novobiocin. Pharmazie 1984, 39, 86–91. [Google Scholar]
- Shaker, R.M. Synthesis and reactions of some new 4H-pyrano[3,2-c]benzopyran-5-one derivatives and their potential biological activities. Pharmazie 1996, 51, 148. [Google Scholar]
- El-Farargy, A.F. Synthesis and some reactions of 8-tert-butyl-6-hydroxy-4-methylcoumarin. Egypt J. Pharm. Sci. 1991, 32, 625. [Google Scholar]
- Manolov, I.; Danchev, N. D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin. Eur. J. Med. Chem. Chim. Ther. 1995, 30, 531–536. [Google Scholar] [CrossRef]
- Emmanuel-Giota, A. A.; Fylaktakidou, K.C.; Hadjipavlou-Latina, D.J.; Litinas, K.E.; Nicolaides, D.N. Synthesis and biological evaluation of several 3-(coumarin-4-yl)tetrahydroisoxazole and 3-(coumarin-4-yl)dihydropyrazole derivatives. J. Heterocycl. Chem. 2001, 38, 717–722. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Kennedy, R.O.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Zahradnik, M. The Production and Applications of Fluorescent Brightening Agent; Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Brown, F.C. 4-Thiazolidinones. Chem. Rev. 1961, 61, 463. [Google Scholar] [CrossRef]
- Singh, S.P.; Parmar, S.S.; Raman, K.; Stenberg, V.I. Chemistry and Biological Activity of Thiazolidinones. Chem. Rev. 1981, 81, 175–203. [Google Scholar] [CrossRef]
- Doran, W.J.; Shonle, H.A. Dialkyl thiazolidinones. J. Org. Chem. 1938, 3, 193–197. [Google Scholar]
- Troutman, H.D.; Long, L.M. The synthesis of 2,3-disubstituted-4-thiazolidinones. J. Am. Chem. Soc. 1948, 70, 3436–3439. [Google Scholar] [CrossRef]
- Rout, M.K.; Mahapatra, G.N. 2-β-Naphtylamino-4-thiazolidinone and its derivatives. J. Am. Chem. Soc. 1955, 77, 2427–2428. [Google Scholar] [CrossRef]
- Gaikwad, N. J.; Tripude, R.N. Substituted 4-thiazolidinones as anticonvulsants. Indian Drugs 1994, 31, 593–594. [Google Scholar]
- El-Gendy, Z.; Abdel-Rahman, R.M.; Fawzy, M.M.; Mahmoud, M.B. Biologically active thiazolidinone derived from thiosemicarbazones. J. Ind. Chem. Soc. 1990, 67, 927–929. [Google Scholar]
- Diumo, M.V.; Mazzoni, O.; Piscopo, E.; Calignano, A; Giordano, F.; Bolognese, A. Synthesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 1992, 35, 2910–2912. [Google Scholar]
- Shah, V.; Pant, C.K.; Joshi, P.C. Synthesis and antifungal activity of some bis(2-arylimino-3-yl-thiazolidinones) and bis(1-aryl-3-yl-thiohydantoins). Asian J. Chem. 1993, 5, 83–88. [Google Scholar]
- Mukhatar, S.; Mujeebur, R.V.P.; Ansari, W.H.; Lemiere, G.; De Groot, A.; Dommisse, R. Bifunctional Derivative of p,p’-Dichlorochalcone. Part II. Synthesis of a Novel Compound 2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone. Molecules 1999, 4, 232–237. [Google Scholar] [CrossRef]
- Sattigeri, V.J.; Soni, A.; Singhal, S.; Khan, S.; Pandya, M.; Bhateja, P.; Mathur., T.; Rattan, A.; Khanna, J.M.; Mehta, A. Synthesis and antimicrobial activity of novel thiazolidinones. ARKIVOC 2005, ii, 46–59. [Google Scholar]
- Iyengar, D.S.; Kamalah, J.; Anandalwar, S.M.; Rangappa, K.S.; Prasad, J. Synthesis and Crystal Structure of 2-( 3-pyridyl)-3-(4-methylphenyl)-1,3-thiazolidin-4-one. Anal. Sci. 2005, 21, 217–218. [Google Scholar]
- Moawad, E.B. Synthesis of some new heterocyclic compounds with expected potential biological activity. J. Islam. Acad. Sci. 1989, 2-4, 237–240. [Google Scholar]
- Čačić, M.; Trkovmik, M.; Čačić, F.; Has-Schon, E. Synthesis of N1-Substituted Coumarino[4,3-c]pyrazoles. J. Heterocycl. Chem. 2002, 40, 833–836. [Google Scholar]
- Cacic, M.; Trkovnik, M.; Cacic, F.; Has-Schon, E. Synthesis and Antimicrobial Activity of Some Derivatives of (7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide. Molecules 2006, 11, 134–147. [Google Scholar] [CrossRef]
- Čačić, M.; Trkovnik, M.; Čačić, F.; Has-Schon, E. Synthesis of [2-aryl-6-oxo-6H-chromeno[6,7-d]oxazol-8-yl]-acetic acid ethyl esters. J. Heterocycl. Chem. 2006, 43, 261–266. [Google Scholar]
- Amin, K.M.; Rahman, D.E.A.; Al-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef]
- Elhafez, O.M.A.; El Khrisy, E.E.; Badria, F.; Fathy, A.E.D.M. Synthesis and biological investigations of new thiazolidinone and oxadiazoline coumarin derivatives. Arch. Pharm. Res. 2003, 26, 686–696. [Google Scholar] [CrossRef]
- Manojkumar, P.; Ravi, T.K.; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009, 59, 159–170. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).