The Synthesis of Some Perhydrobenzimidazolinium Salts and Their Application in Pd-Carbene Catalyzed Heck and Suzuki Reactions
Abstract
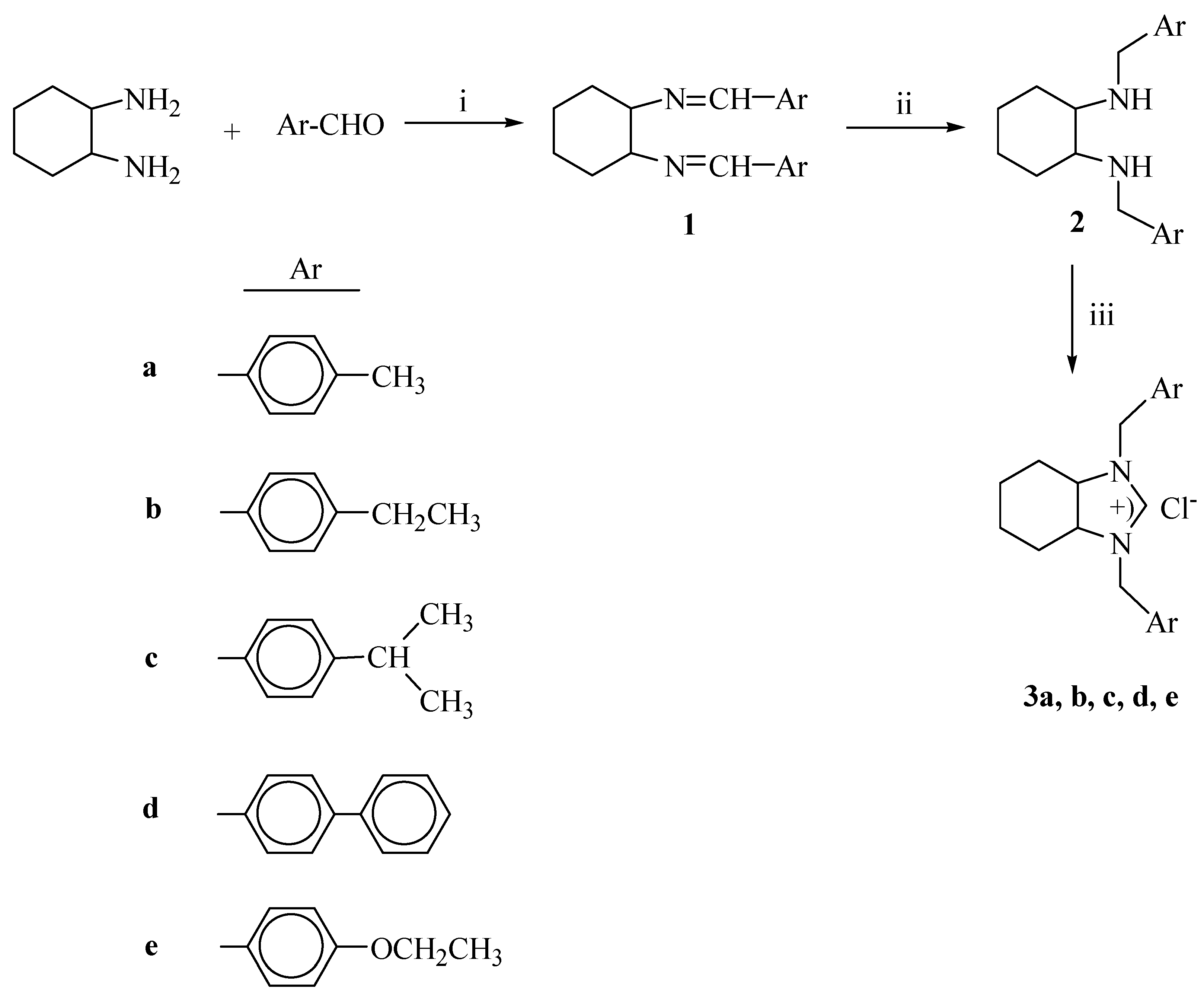
:1. Introduction
2. Results and Discussion
| Entry | R | SALT | Yield (%)b,c |
|---|---|---|---|
| 1 | COCH3 | 3a | 96 |
| 2 | COCH3 | 3b | 95 |
| 3 | COCH3 | 3c | 98 |
| 4 | COCH3 | 3d | 98 |
| 5 | COCH3 | 3e | 96 |
| 6 | CHO | 3a | 95 |
| 7 | CHO | 3b | 94 |
| 8 | CHO | 3c | 96 |
| 9 | CHO | 3d | 91 |
| 10 | CHO | 3e | 97 |
| 11 | H | 3a | 94 |
| 12 | H | 3b | 93 |
| 13 | H | 3c | 90 |
| 14 | H | 3d | 92 |
| 15 | H | 3e | 95 |
| 16 | OCH3 | 3a | 92 |
| 17 | OCH3 | 3b | 88 |
| 18 | OCH3 | 3c | 93 |
| 19 | OCH3 | 3d | 91 |
| 20 | OCH3 | 3e | 87 |
| 21 | CH3 | 3a | 86 |
| 22 | CH3 | 3b | 89 |
| 23 | CH3 | 3c | 85 |
| 24 | CH3 | 3d | 81 |
| 25 | CH3 | 3e | 84 |
| Entry | R | SALT | Yield (%)b,c |
|---|---|---|---|
| 1 | COCH3 | 3a | 94 |
| 2 | COCH3 | 3b | 95 |
| 3 | COCH3 | 3c | 97 |
| 4 | COCH3 | 3d | 98 |
| 5 | COCH3 | 3e | 92 |
| 6 | CHO | 3a | 93 |
| 7 | CHO | 3b | 97 |
| 8 | CHO | 3c | 98 |
| 9 | CHO | 3d | 94 |
| 10 | CHO | 3e | 91 |
| 11 | H | 3a | 87 |
| 12 | H | 3b | 89 |
| 13 | H | 3c | 86 |
| 14 | H | 3d | 94 |
| 15 | H | 3e | 90 |
| 16 | OCH3 | 3a | 84 |
| 17 | OCH3 | 3b | 86 |
| 18 | OCH3 | 3c | 88 |
| 19 | OCH3 | 3d | 89 |
| 20 | OCH3 | 3e | 82 |
| 21 | CH3 | 3a | 78 |
| 22 | CH3 | 3b | 75 |
| 23 | CH3 | 3c | 81 |
| 24 | CH3 | 3d | 77 |
| 25 | CH3 | 3e | 74 |
3. Conclusions
4. Experimental
4.1. General
4.2. General procedure for preparation of the 1,3-dialkylperhydrobenzimidazolinium chlorides 3a-e
4.3. General procedure for the Heck coupling reactions
4.4. General procedure for the Suzuki coupling reactions
Acknowledgements
References
- Trost, B.M.; Verhoeven, T.R. Organopalladium Compounds in Organic Synthesis and in Catalysis. In Comprehensive Organometallic Chemistry; Wilkinson, G., Stone, F.G.A., Abel, E.W., Eds.; Pergamon: Oxford, UK, 1982; Volume 8, pp. 799–938. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Tietze, L.F.; Petersen, S. Stereoselective Synthesis of Novel 19-Nor-Steroids by a Double Heck Reaction. Eur. J. Org. Chem. 2001, 9, 1619–1624. [Google Scholar] [CrossRef]
- Cianga, I.; Yağci, Y. Synthesis and Characterization of Comb-Like Polyphenylenes via Suzuki Coupling of Polystyrene Macromonomers Prepared by Atom Transfer Radical Polymerization. Eur. Polymer J. 2002, 38, 695–703. [Google Scholar] [CrossRef]
- Busacca, C.A.; Eriksson, M.C.; Fiaschi, R. Cross Coupling of Vinyl Triflates and Alkyl Grignard Reagents Catalyzed by Nickel(0)-Complexes. Tetrahedron Lett. 1999, 40, 3101–3104. [Google Scholar] [CrossRef]
- Miller, J.A.; Farrell, R.P. Synthesis of Functionally Substituted Unsymmetrical Biaryls via a Novel Double Metal Catalyzed Coupling Reaction. Tetrahedron Lett. 1998, 39, 7275–7278. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions via Organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.; Xiao, J. Palladium Catalyzed Regioselective Arylation of Electron-Rich Olefins by Aryl Halides. J. Mol. Catal. A 2012, 187, 189–193. [Google Scholar]
- Jafarpour, L.; Nolan, S.P. Transition-Metal Systems Bearing a NucleophilicCarbene Ancillary Ligand: From Thermochemistry to Catalysis. Adv. Organomet. Chem. 2000, 46, 181–222. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Recent Homogeneous Catalytic Applications of Chelate and Pincer N-Heterocyclic Carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Sluys, M.V.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki-Heck and Suzuki-Miyaura Couplings - Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Casalnuova, A.L.; Calabrese, J.C. Palladium-Catalyzed Alkylations in Aqueous Media. J. Am. Chem. Soc. 1990, 112, 4324–4330. [Google Scholar] [CrossRef]
- Churruca, F.; SanMartin, R.; İnes, B.; tellitu, I.; Dominquez, E. Hydrophilic CNC-Pincer Palladium Complexes: A Source for Highyl Efficient, Recyclable Homogeneous Catalysts in Suzuki-Miyaura Cross-Coupling. Adv. Synth. Catal. 2006, 348, 1836–1840. [Google Scholar] [CrossRef]
- Alimardanov, A.; Schmieder-Van de Vondervoort, L.; de Vries, A.H.M.; de Vries, J.G. Use of “Homeopathic” Ligand-Free Palladium as Catalyst for Aryl-Aryl Coupling Reactions. Adv. Synth. Catal. 2004, 346, 1812–1817. [Google Scholar] [CrossRef]
- de Vries, J.G. A Unifying Mechanism for all High-Temperature Heck Reactions. The Role of Palladium Colloids and Anionic Species. Dalton Trans. 2006, 421–429. [Google Scholar] [CrossRef]
- Saha, D.; Chattopadhyay, K.; Ranu, B.C. Aerobic Ligand-Free Suzuki Coupling Catalyzed by in Situ-Generated Palladium Nanoparticles in Water. Tetrahedron Lett. 2009, 50, 1003–1006. [Google Scholar] [CrossRef]
- Li, C.J.; Chan, T.H. Organic Reactions in Aqueous Media; John Wiley & Sons: New York, 1997. [Google Scholar]
- Genet, J.P.; Savignac, M. Recent Developments of Palladium(0) Catalyzed Reactions in Aqueous Medium. J. Organomet. Chem. 1999, 576, 305–317. [Google Scholar] [CrossRef]
- Sakurai, H.; Tsukuda, T.; Hirao, T. Pd/C as a Reusable Catalyst for the Coupling Reaction of Halophenols and Arylboronic Acids in Aqueous Media. J. Org. Chem. 2002, 67, 2721–2722. [Google Scholar] [CrossRef]
- Parisot, S.; Kolodziuk, R.; Henry, C.G.; Iourtchenko, A.; Sinou, D. Glucosamine-Based Phosphines. Efficient Ligands in the Suzuki Cross-Coupling Reaction in Water. Tetrahedron Lett. 2002, 43, 7397–7400. [Google Scholar] [CrossRef]
- Schönfelder, D.; Nuyken, O.; Weberskirch, R. Heck and Suzuki Coupling Reactions in Water Using Poly(2-Oxazoline)s Functionalized with Palladium Carbene Complexes as Soluble, Amphiphilic Polymer Supports. J. Organomet. Chem. 2005, 690, 4648–4655. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, X.; Tu, C.; Li, X.; Sun, H. Aqueous Suzuki Coupling Reaction Catalyzed by Water-Soluble Diimine/Pd(II) Systems. J. Organomet. Chem. 2009, 694, 697–702. [Google Scholar] [CrossRef]
- Ma, J.; Cui, X.; Gao, L.; Wu, Y. FerrocenylimidazolinePalladacycles: Syntheses, Crystal Structures and Applications as Catalysts for Suzuki Cross Coupling Reaction in Water. Inorg. Chem. Commun. 2007, 10, 762–766. [Google Scholar] [CrossRef]
- Shang, Y.J.; Wu, J.W.; Fan, C.L.; Hu, J.S.; Lu, B.Y. Synthesis of 1,3-Bis-(5-Ferrocenylisoxazole-3-yl)Benzene-Derived Palladium(II)acetate Complex and its Application in Mizoroki–Heck Reaction in an Aqueous Solution. J. Organomet. Chem. 2008, 693, 2963–2966. [Google Scholar] [CrossRef]
- Vashchenko, V.; Krivoshey, A.; Knyazeva, I.; Petrenko, A.; Goodby, J.W. Palladium-Catalyzed Suzuki Cross-Coupling Reactions in a Microemulsion. Tetrahedron Lett. 2008, 49, 1445–1449. [Google Scholar] [CrossRef]
- Kostas, I.D.; Coutsolelos, A.G.; Charalambidis, G.; Skondrab, A. The First Use of Porphyrins as Catalysts in Cross-Coupling Reactions: A Water-Soluble Palladium Complex with a PorphyrinLigand as an Efficient Catalyst Precursor for the Suzuki-Miyaura Reaction in Aqueous Media under Aerobic Conditions. Tetrahedron Lett. 2007, 48, 6688–6691. [Google Scholar] [CrossRef]
- Shaughnessy, K.H.; Booth, R.S. Sterically Demanding, Water-Soluble Alkylphosphines as Ligands for High Activity Suzuki Coupling of Aryl Bromides in Aqueous Solvents. Org. Lett. 2001, 3, 2757–2759. [Google Scholar] [CrossRef]
- Moore, L.R.; Shaughnessy, K.H. Efficient Aqueous-Phase Heck and Suzuki Couplings of Aryl Bromides Using Tri(4,6-dimethyl-3- sulfonatophenyl)phosphine Trisodium Salt (TXPTS). Org. Lett. 2004, 6, 225–228. [Google Scholar] [CrossRef]
- Özdemir, İ.; Yiğit, M.; Çetinkaya, E.; Çetinkaya, B. Synthesis of Novel Palladium N-Heterocyclic-Carbene Complexes as Catalysts for Heck and Suzuki Cross-Coupling Reactions. Appl. Organometal. Chem. 2006, 20, 187–192. [Google Scholar] [CrossRef]
- Yiğit, M.; Yiğit, B.; Özdemir, İ.; Çetinkaya, E.; Çetinkaya, B. Active Ruthenium-(N-Heterocyclic Carbene) Complexes for Hydrogenation of Ketones. Appl. Organometal. Chem. 2006, 20, 322–327. [Google Scholar] [CrossRef]
- Yiğit, M.; Özdemir, İ.; Çetinkaya, E.; Çetinkaya, B. Novel Rhodium N-Heterocyclic Carbene Catalysed Arylation of Aldehydes with Phenylboronic acid. Trans. Met. Chem. 2007, 32, 536–540. [Google Scholar] [CrossRef]
- Yiğit, M.; Özdemir, İ.; Çetinkaya, E.; Çetinkaya, B. In Situ Generated Rhodium-Based Catalyst for Addition of Phenylboronic Acid to Aldehydes. Heteroatom Chem. 2005, 16, 461–465. [Google Scholar] [CrossRef]
- Yiğit, M.; Özdemir, İ.; Çetinkaya, B.; Çetinkaya, E. Novel N-heterocyclic-Carbene-Rhodium Complexes as Hydrosilylation Catalysts. J. Mol. Catal. A 2005, 241, 88–92. [Google Scholar] [CrossRef]
- Yaşar, S.; Özdemir, İ.; Çetinkaya, B.; Renaud , J.L.; Bruneau, C. Benzylic İmidazolidinium, 3,4,5,6-Tetrahydropyrimidinium and Benzimidazolium Salts: Applications in Ruthenium-Catalyzed Allylic Substitution Reactions. Eur. J. Org. Chem. 2008, 2142–2149. [Google Scholar]
- Huang, W.; Guo, J.; Xiao, Y.; Zhu, M.; Zou, G.; Tang, J. Palladium-Benzimidazolium Salt Catalyst Systems for Suzuki Coupling: Development of a Practical and Highly Active Palladium Catalyst System for Coupling of Aromatic Halides with Arylboronic Acids. Tetrahedron 2005, 61, 9783–9790. [Google Scholar] [CrossRef]
- Özdemir, İ.; Gök, Y.; Gürbüz, N.; Çetinkaya, B. Benzimidazolylidene Carbene Ligated Palladium Catalysis of the Heck Reaction in Aqueous Media. Turk. J. Chem. 2007, 31, 397–402. [Google Scholar]
- Grasa, G.A.; Viciu, M.S.; Huang, J.; Zhang, C.; Trudell, M.L.; Nolan, S.P. Suzuki-Miyaura Cross-Coupling Reactions Mediated by Palladium/İmidazolium Salt Systems. Organometallics 2002, 21, 2866. [Google Scholar] [CrossRef]
- Ozdemir, İ.; Demir, S.; Çetinkaya, B. Palladium-Catalyzed Heck Reaction of Aryl Bromides in Aqueous Media Using Tris(N-Heterocyclic Carbene) Ligands. Synlett. 2007, 6, 889–892. [Google Scholar] [CrossRef]
- Loch, J.A.; Albrecht, M.; Peris, E.; Mata, J.; Faller, J.W.; Crabtree, R.H. Palladium Complexes with Tridentate Pincer Bis-CarbeneLigands as Efficient Catalysts for C−C Coupling. Organometallics 2002, 21, 700–706. [Google Scholar] [CrossRef]
- Baillie, C.; Xiao, J.L. Palladium-Catalysed Synthesis of BiarylPhosphines. Tetrahedron 2004, 60, 4159–4168. [Google Scholar] [CrossRef]
- Zhang, C.M.; Huang, J.K.; Trudell, M.L.; Nolan, S.P. Palladium−Imidazol-2-ylidene Complexes as Catalysts for Facile and Efficient Suzuki Cross-Coupling Reactions of Aryl Chlorides with Arylboronic Acids. J. Org. Chem. 1999, 64, 3804–3805. [Google Scholar] [CrossRef]
- Altenhoff, G.; Goddart, R.; Lehmann, C.W.; Glorius, F. Sterically Demanding, Bioxazoline-Derived N-Heterocyclic CarbeneLigands with Restricted Flexibility for Catalysis. J. Am. Chem. Soc. 2004, 126, 15195–15201. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yiğit, M. The Synthesis of Some Perhydrobenzimidazolinium Salts and Their Application in Pd-Carbene Catalyzed Heck and Suzuki Reactions. Molecules 2009, 14, 2032-2042. https://doi.org/10.3390/molecules14062032
Yiğit M. The Synthesis of Some Perhydrobenzimidazolinium Salts and Their Application in Pd-Carbene Catalyzed Heck and Suzuki Reactions. Molecules. 2009; 14(6):2032-2042. https://doi.org/10.3390/molecules14062032
Chicago/Turabian StyleYiğit, Murat. 2009. "The Synthesis of Some Perhydrobenzimidazolinium Salts and Their Application in Pd-Carbene Catalyzed Heck and Suzuki Reactions" Molecules 14, no. 6: 2032-2042. https://doi.org/10.3390/molecules14062032







